Successful Implementation of GS1 Standards Step-by-Step Approach
75 Slides5.10 MB

Successful Implementation of GS1 Standards Step-by-Step Approach Salil Joshi, Senior Director – Industry Development GS1 US October 19, 2016

Benefits of Standards KEY BENEFITS Improving patient safety Lowering costs through increased efficiency Reducing medication errors Enabling supply chain visibility Facilitating effective product recalls Tracking of pharmaceutical products/medical devices Reducing introduction of counterfeit products Enhancing inventory management Linking critical product data to the patient record Supporting regulatory compliance Optimizing order, invoice, sales reporting, and chargeback/rebate processes 2016 GS1 US All Rights Reserved 15

GS1 Standards in Healthcare 2016 GS1 US All Rights Reserved 16

We envision a world where The supply chain delivers: the right product to the right place at the right time Healthcare professionals spend their time providing care: to the right patient administering the right dose through the right route Focus is on reducing error from the process with automation and the use of standard, unique identifiers. Regulatory efforts since the 1999 Institute of Medicine report To Error is Human focused on marking products to enable automation and further visibility to protect patients in the event of a product recall. 2016 GS1 US All Rights Reserved 17

Regulations Driving Change 2016 GS1 US All Rights Reserved 18

When Will We See Usage Increase? “When should a Hospital Provider begin preparing to meet upcoming regulations?” sh r flu ry as Ba o t ven ce h II In Devi s las Med es IC UD ity of Cod jo r Ma s las IC D U In III v sI las C I UD us h y fl r o e nt In v DSCSA 2023 CMS – Reimbursement 2021 DSCSA 2019 Meaningful Use Stage 2 2015 – 2017 ONC 2018 & UDI Class I sh flu y r to en UDI Class II 09/24/2016 Meaningful Use Stage 2018 & beyond DSCSA 2017 Today UDI Class III 2014 Usage of standards increases “Backward scheduling would suggest need to start in late 2016 early 2017 assuming 12 to 18 month timeline” Build the foundation for interoperability and adoption 2016 - 2017 2016 GS1 US All Rights Reserved 19

Regulations Driving Change The FDA Unique Device Identification (UDI) Rule and Drug Supply Chain Security Act (DSCSA) are being implemented Packaging Changes for Products Serialization Requirements for drugs Track and Trace Initiative in Rx EHR Regulation – Meaningful Use and EHR Certification Proposed Rules from ONC 2016 GS1 US All Rights Reserved 20
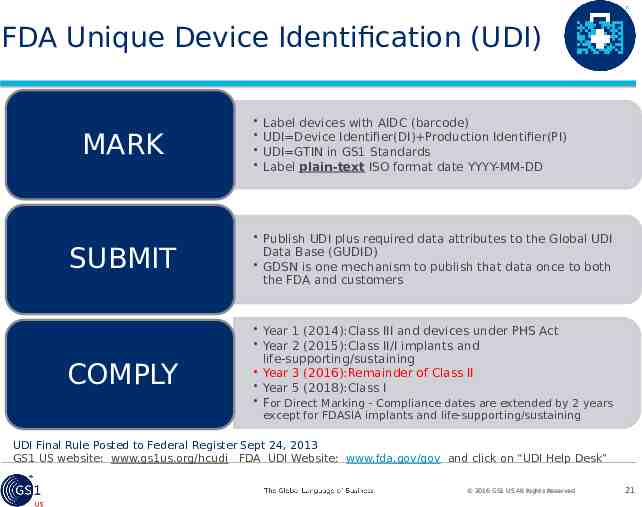
FDA Unique Device Identification (UDI) MARK SUBMIT COMPLY Label devices with AIDC (barcode) UDI Device Identifier(DI) Production Identifier(PI) UDI GTIN in GS1 Standards Label plain-text ISO format date YYYY-MM-DD Publish UDI plus required data attributes to the Global UDI Data Base (GUDID) GDSN is one mechanism to publish that data once to both the FDA and customers Year 1 (2014):Class III and devices under PHS Act Year 2 (2015):Class II/I implants and life-supporting/sustaining Year 3 (2016):Remainder of Class II Year 5 (2018):Class I For Direct Marking - Compliance dates are extended by 2 years except for FDASIA implants and life-supporting/sustaining UDI Final Rule Posted to Federal Register Sept 24, 2013 GS1 US website: www.gs1us.org/hcudi FDA UDI Website: www.fda.gov/gov and click on “UDI Help Desk” 2016 GS1 US All Rights Reserved 21

US FDA UDI General Rule The label* of EVERY medical device (including all IVDs) must have a UDI. EVERY device package (contains a fixed quantity of a version or model) must have a UDI. Any other approach is an exception to or alternative from these requirements. * Section 201(k) defines 'label' as a display of written, printed, or graphic matter upon the immediate container of any article. 2016 GS1 US All Rights Reserved 22

GS1 US and UDI GS1 has been accredited by the FDA as an Issuing Agency for the assignment of UDIs in the context of the U.S. FDA Unique Device Identification System, and GS1 US serves as the first point of contact for the FDA. As of February 2016, about 90% of the products in GUDID have been assigned GTINs. 2016 GS1 US All Rights Reserved 23

Global Unique Device Identifier Database (GUDID) Website Operated by the FDA to collect information on Medical Devices 2016 GS1 US All Rights Reserved 24

GUDID Global Device Identifier Database (GUDID) Operated by the FDA to collect information on Medical Devices Public facing website to share data with anyone ( https://accessgudid.nlm.nih.gov) Some information submitted to the GUDID will be private (for FDA only) All medical devices which are regulated under the UDI regulations will be required to be listed in the GUDID Sunrise for each class is the same for GUDID as assigning a UDI Class III devices- 1 year from final rule publication (September 24, 2014) Class II “life sustaining” devices- 2 years from final rule publication (September 24, 2015) Class II remaining devices- 3 years from final rule publication (September 24, 2016) Class I devices- 5 years from final rule publication (September 24, 2018) 2016 GS1 US All Rights Reserved 25

GUDID Loading Data loading responsibility lies with the “Labeler” FDA defines the “Labeler” as the entity responsible for the contents of the label In GS1 speak, this is the Brand Owner Data is loaded for the Device Identifier (DI) Only UDI is a Device Identifier (DI) and Production Identifier (PI) Only the DI is used in the GUDID Data provided is master data and is used for public consumption, however there are some elements which are for FDA only 2016 GS1 US All Rights Reserved 26

UDI Components UDI Device Identifier (DI) Production Identifiers (PI) GTIN Application Identifiers (AI) GTIN – Global Trade Item Number Lot Number Serial Number Expiry Date 2016 GS1 US All Rights Reserved 27

How is UDI documented? The product is scanned, the data is sent to the EHR, which parses the data to the respective data fields 2016 GS1 US All Rights Reserved 28

Benefits of UDI Helps providers identify patients implanted with recalled products using a standardized identifier Enables patients and providers to submit more precise adverse event reports Supports care coordination by providing physicians with precise information on the devices implanted in patients Allows hospitals to perform analyses comparing devices used in that facility Allows providers to submit information to device registries Provides a foundation to address counterfeiting of products Source: http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/UniqueDeviceIdentification/ BenefitsofaUDIsystem/default.htm 2016 GS1 US All Rights Reserved 29

Provider Steps for UDI Implementation Step 1: Establish Executive Support Step 2: Form a UDI Implementation Team (Clinical, Supply Chain, IT) Step 3: Develop Project Communication Internal and External with suppliers Step 4: Assess Information Systems (ERP and EHR) Step 5: Identify/Obtain UDI Product Data Select source (GDSN, GUDID, GPO, Supplier) Step 6: Engage Suppliers for Pilot & Testing Step 7: Conduct Transactional Testing Step 8: Create Standard Operating Procedures 2016 GS1 US All Rights Reserved 30

Successful Implementation of GS1 Standards What Does It Mean for St. Joseph Health?

St. Joseph Health ABOUT US Not-For-Profit Integrated Catholic Health Care Delivery System based in Irvine, California Founded by the Sisters of St. Joseph of Orange First Hospital established in 1920 in Eureka, California Health System established in 1982

St. Joseph Health Three Geographic Regions: Northern California, Southern California and Texas/Eastern New Mexico Total Revenue of 5.6 Billion 16 hospitals, as well as home health agencies, hospice care, outpatient services, skilled nursing facilities and physician organizations Nearly 24,000 employees and more than 1,500 affiliated physicians Supply Chain spend is 850 million Centralized Purchasing/AP

St. Joseph Health Our Mission To extend the healing ministry of Jesus in the tradition of the Sisters of St. Joseph of Orange by continually improving the health and quality of life of people in the communities we serve Our Vision To bring people together to provide compassionate care, promote health improvement and create healthy communities

Why GS1 Standards Adoption?

Benefits of GS1 Standards Item Master standardization Analytics Patient Safety EMR Electronic transactions (EDI) Reform and Regulations

GS1 Healthcare House

What was our GS1 Standards implementation process?

Engaging GS1US In Jan. 2016, SJH asked GS1US to include us in a GS1 implementation plan, including: – Documenting the process of implementation – Tracking ROI

Outside Consumtant Outside consulting firm assisted us with implementing GS1 Standards more quickly and seamlessly, so we can better support efficient and safe patient care. They assisted us in preparing for our GS1 Standards implementation through: – Cleaning up GLNs – Boarding GLN with GHX – Transacting using both GLN and GTIN – Documenting Step by step business process form maintaining GS1 Standards after go live.

GLN Clean-Up SJH began the clean-up of our Global Location Number (GLN) and boarding process with GHX: Clean-up of “ship-to” locations within PMM Request new GLN when needed – Currently we must go through GPO, but we are working on bringing our GLN maintenance in-house

GLN & GTIN Testing In May 2016, SJH began testing the Global Location Number (GLN) and Global Trade Item Number (GTIN) transaction with vendors Cook Medical and Abbott By the end of May, we had our first complete transaction (850, 855, 810)

What are the next steps?

Next Steps 1. Load GLN into PMM for all “ship-to” locations (not including VL at this time) so that we can begin using GLN to transact with both Cook Medical and Abbott 2. Load GTIN into PMM 3. Requesting GTIN information with new contract price files 4. Identify data pool company 5. Begin testing process with Johnson & Johnson 6. Track progress

Communications Communication/education with Ministry Supply Chain Directors Communication explaining GS1 Standards in The Huddle for System Office and Ministries Management Minute article Communications to Exec Leadership, as needed, including Momentum Newsletter & Chatter Conference Presentation White Paper

Glossary GLN - Global Location Number GTIN - Global Trade Item Number GDSN - Global Data Synchronization Network UDI - Unique Device Identification GUDID - Global UDI Database

Questions?
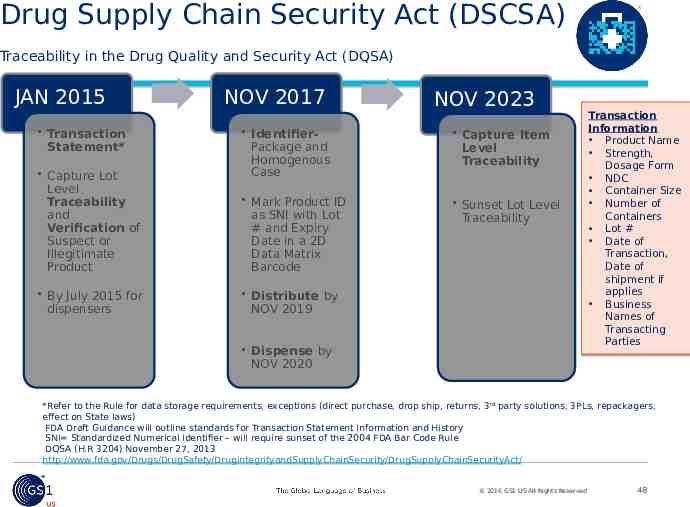
Drug Supply Chain Security Act (DSCSA) Traceability in the Drug Quality and Security Act (DQSA) JAN 2015 Transaction Statement* Capture Lot Level Traceability and Verification of Suspect or Illegitimate Product By July 2015 for dispensers NOV 2017 NOV 2023 IdentifierPackage and Homogenous Case Capture Item Level Traceability Mark Product ID as SNI with Lot # and Expiry Date in a 2D Data Matrix Barcode Sunset Lot Level Traceability Distribute by NOV 2019 Dispense by NOV 2020 Transaction Information Product Name Strength, Dosage Form NDC Container Size Number of Containers Lot # Date of Transaction, Date of shipment if applies Business Names of Transacting Parties *Refer to the Rule for data storage requirements, exceptions (direct purchase, drop ship, returns, 3rd party solutions, 3PLs, repackagers, effect on State laws) FDA Draft Guidance will outline standards for Transaction Statement Information and History SNI Standardized Numerical Identifier – will require sunset of the 2004 FDA Bar Code Rule DQSA (H.R 3204) November 27, 2013 http://www.fda.gov/Drugs/DrugSafety/DrugIntegrityandSupplyChainSecurity/DrugSupplyChainSecurityAct/ 2016 GS1 US All Rights Reserved 48

GS1 Standards in Healthcare GS1 Standards for identifying, capturing, and sharing information— about products, business locations, and more — make it possible for companies to speak the same language, connect with each other, and move their business forward. 2016 GS1 US All Rights Reserved 49

GS1 System of Standards 2016 GS1 US All Rights Reserved 50

What Is The GS1 System? GLN Global Location Number – Location Identification GTIN Global Trade Item Number – Product Identification GDSN Global Data Synchronization Network – Data Sharing and Attribute Accuracy 2016 GS1 US All Rights Reserved 51

IDENTIFY: GS1 Identifiers GS1 Standards begin with GS1 Identification Numbers used to uniquely distinguish all products (trade items), logistic units, locations, assets, and relationships across the supply chain from manufacturer to consumer. 2016 GS1 US All Rights Reserved 52
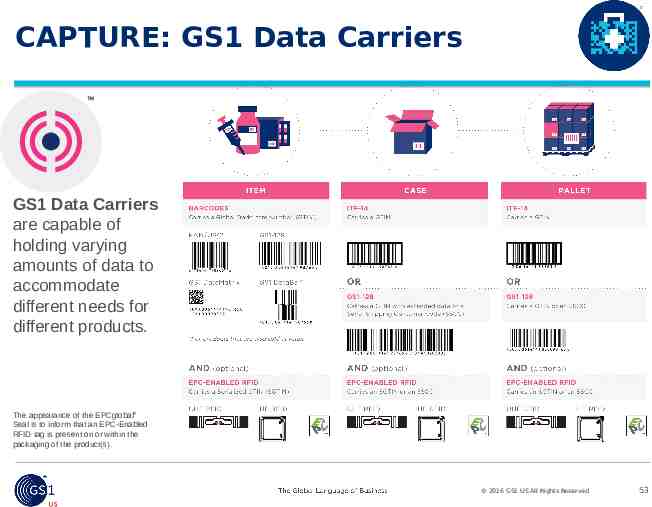
CAPTURE: GS1 Data Carriers GS1 Data Carriers are capable of holding varying amounts of data to accommodate different needs for different products. The appearance of the EPCglobal Seal is to inform that an EPC-Enabled RFID tag is present on or within the packaging of the product(s). 2016 GS1 US All Rights Reserved 53

SHARE: GS1 Data Exchange Interoperability, made possible by identification standards, data capture standards, and data exchange standards, allows product information to flow through the supply chain. 2016 GS1 US All Rights Reserved 54

United Nations Standard Products & Services Code (UNSPSC ) An open, global, multi-industry standard used to classify products and services Provides a structure or framework for product classification Provides a way to categorize products and services to help users organize products for catalogs, databases and listings Owned by: United Nations Development Program (UNDP) Managed by GS1 US since May 2003 Designed to facilitate electronic commerce Help streamline the procurement process Help analyze spending Help customers find products 2016 GS1 US All Rights Reserved 55

Global Location Number (GLN) 2016 GS1 US All Rights Reserved 56

Location Identification in Healthcare Too many identifiers for the same healthcare location Confusion & inefficiency SAINT JOHN'S QUEENS HOSPITAL 1100004570208 ST JOHN'S QUEENS HOSPITAL 100084547 SAINT JOHNS QUEENS HOSPITAL JAOE SAINT JOHN'S QUEEN HOSPITAL 50003000431 SAINT JOHN'S QUEEN’S HOSPITAL CA2053 Many different names and location numbers for same hospital location ST. JOHN'S QUEENS HOSPITAL OM 12345 2016 GS1 US All Rights Reserved 57

Global Location Number Overview The use of standardized location identification not only ensures that the right product arrives at the right place at the right time, it also enables more efficient business practices and helps to drive down supply chain costs Basically the GLN points to a number in a database that references the following criteria: Who, What and Where Who controls the GLN; What qualifies or states the context of the relationship of the related data; and Where is the physical address 2016 GS1 US All Rights Reserved 58

What is a Global Location Number (GLN)? A globally unique 13-digit number that identifies: Legal entities (whole companies, health systems, divisions) Functional entities or specific department within a legal entity (Pharmacy, receiving department, accounts payable) Physical locations (Clinics, units, warehouses, loading docks, room/shelf locations) 2016 GS1 US All Rights Reserved 59

GLN Hierarchies – Getting Started Organizations can assign GLN’s to various supply chain locations in their organization to facilitate precise location identification in a business Questions to consider Who is the buying organization? Where will the products be shipped/received? Who will receive the invoice? Who is the selling organization? Where are the payments sent? 2016 GS1 US All Rights Reserved 60

Provider GLN Assignment Assign a GLN to the main entity (DONE) Check with GLN Registry to see if a GLN has been assigned to this level Assign a number to each specific location within the company (depending on your need) (DONE) Requires a GS1 Company Prefix Do NOT build intelligence into the number Use the GLN registry to communicate GLNs and structure to suppliers 2016 GS1 US All Rights Reserved 61

Provider to set up Vendors Vendors (Manufacturers/Distributors) should be enumerated with GLNs for use in Ordering Invoicing Payments Providers need to store these numbers in their Vendor Master files Communicate hospital GLN hierarchy and numbers to vendors 2016 GS1 US All Rights Reserved 62

GLN Hierarchy 2016 GS1 US All Rights Reserved 63

GLN Hierarchy 2016 GS1 US All Rights Reserved 64

GLN Structure Example Note: Use the Check Digit Calculator on the GS1 US website http://www.gs1us.org/resources/tools/check-digit-calculator 2016 GS1 US All Rights Reserved 65

How are GLNs USED? A GLN may be encoded within a barcode for specific business applications and placed on shipping labels (help users to ensure that each GLN is specific to one unique location within the world instead of phone numbers, store numbers, DUNS 4): “Ship to – deliver to” locations “Bill to – invoice to” locations “Purchased from” locations Shelf locations GLNs are used in eCommerce EDI and XML GLNs are required for Global Data Synchronization Identifies parties and locations in order to match records from a data pool 2016 GS1 US All Rights Reserved 66

GLN Benefits for Providers Simplifies location identification with a single identifier used across all supply chain partners Enables providers to define and manage their own account and location information to ensure accuracy Provides an accurate view of the organization as a customer to ensure correct contract pricing eligibility and to streamline rebate processing Reduces mis-shipments and time spent resolving order and invoice errors Provides the foundation for traceability to improve product recall processes 2016 GS1 US All Rights Reserved 67

Next Steps for GLN Implementation Register for GLN Registry Training Take ownership of your GLN Registry Records Contact your GPO or GS1 US for your GLN hierarchy Review and Update your GLN Registry Records Synchronize GLNs with Trading Partners Conduct Pilot Project and Test GLNs in Transactions Repeat with next supplier Ongoing Maintenance 2016 GS1 US All Rights Reserved 68

Global Trade Item Number (GTIN) 2016 GS1 US All Rights Reserved 69

Issue #1 - Problem with Item Identification Manufacturer Product # 383520 Distributor Product # 0723383520 Hospital Product # 1114985 (GTIN 00382903835201) Artwork Courtesy of GS1 and BD 2016 GS1 US All Rights Reserved 70

Issue #2: Catalog Numbers Not Unique Cat # BD Product - 14 G x 3.25 in. BD 382268 382268 Angiocath peripheral venous Other Supplier Dentsply 03-822-68 SS GLD 018X022 14IN PKG 10 catheter (2.1 mm x 83 mm) made of FEP polymer. (10/sp, 50/ca) 381705 381705 - 18 G x 1.16 in. BD Angiocath Autoguard shielded IV catheter (1.3 mm x 30 mm) made of FEP polymer. (50/sp, 200/ca) Mallinckrodt 3817-05 CHEMICAL DRY SODIUM PHOSPHATE DIBASIC 371073 371073 - BD E-Z Scrub surgical scrub brush impregnated with 4% CHG. Color coded red. (30/sp, 300/ca) Codman 371073 DEBAKEY BULLDOG CLAMP RING HANDLE ANGLED 90? STRAIGHT SHAFT 41MM JAW 4 3/4'' (120MM) 305905 305905 - 3 mL BD SafetyGlide syringe with 23 G x 1 in. shielding intramuscular injection needle, regular bevel, regular wall. Detachable needle. (50/sp, 400/ca) CARL ZEISS 305905 FLOORSTAND S-1 COMPLETE, W/ARTICULATED ARM SYSTEM FOR OPMI & ACCESS 2.5-7 KG. 2016 GS1 US All Rights Reserved 71

Issue #3: Trading Partner Numbers (329461 - 1/2 mL BD Lo-Dose U-100 Insulin Syringe) Business Name Item Number Type Item Number BD Mfg Catalog Number 329461 BD GTIN 00382903294619 BD GTIN 30382903294610 BD GTIN 50382903294614 CARDINAL HEALTH PV Order Number BF329461 OWENS & MINOR PV Order Number 0722329461 OWENS & MINOR PV Order Number 0723329461 AMERICAN MEDICAL DEPOT Vendor Catalog Number 777127217 AMERICAN MEDICAL DEPOT Vendor Catalog Number 777127218 GOVERNMENT SCI SOURCE Vendor Catalog Number FSC1482679CS GOVERNMENT SCI SOURCE Vendor Catalog Number FSC1482679PK ALLIANCE JOINT VENTURE Vendor Catalog Number 888021932 THOMAS SCIENTIFIC Vendor Catalog Number 8938M25 THOMAS SCIENTIFIC Vendor Catalog Number 8938M28 VWR INTERNATIONAL Vendor Catalog Number BD329461 2016 GS1 US All Rights Reserved 72

Solution – Global Trade Item Number A unique 8, 12, 13, or 14-digit number identifies products and services of the GS1 System, as an unambiguous global standard for collaborative commerce A 14-digit GTIN can be encoded as a GS1-128 / GS1 DataMatrix / GS1 DataBar / EPC/RFID GS1-128 (14-digit GTIN) DataMatrix (14-digit GTIN) Databar (14-digit GTIN) EPC/RFID 2016 GS1 US All Rights Reserved 73

GTIN Introduction The Global Trade Item Number (GTIN) is an identification key in the GS1 System of Standards. The GTIN is used for uniquely identifying trade items, which includes both products and services that are priced, ordered, and invoiced at any point in the supply chain worldwide. GTINs are placed on trade items as barcodes: GTIN A pointer that identifies product characteristics Always numeric and to be stored as text Barcode Symbology that carries the GTIN A non-intelligent number Allocated according to the GTIN ALLOCATION RULES 2016 GS1 US All Rights Reserved 74

GTIN for Each Packaging Level 2016 GS1 US All Rights Reserved 75
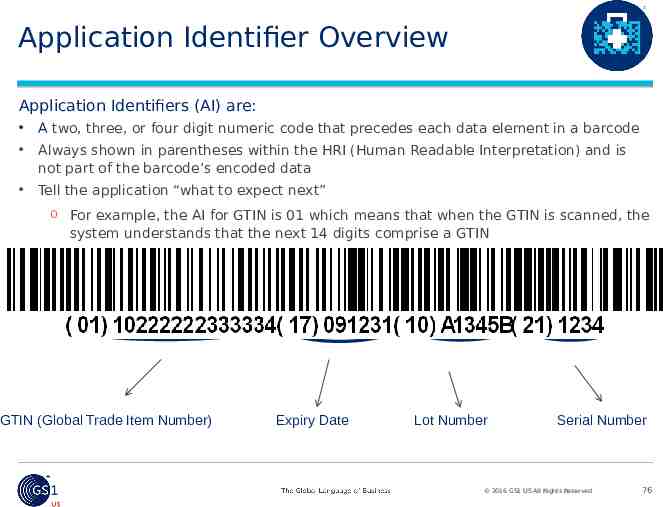
Application Identifier Overview Application Identifiers (AI) are: A two, three, or four digit numeric code that precedes each data element in a barcode Always shown in parentheses within the HRI (Human Readable Interpretation) and is not part of the barcode’s encoded data Tell the application “what to expect next” o For example, the AI for GTIN is 01 which means that when the GTIN is scanned, the system understands that the next 14 digits comprise a GTIN GTIN (Global Trade Item Number) Expiry Date Lot Number Serial Number 2016 GS1 US All Rights Reserved 76

Application Identifier Overview Application Identifiers provide the ability to string together multiple fields of information to help solve a variety of business challenges. The AIs are a finite set of specialized identifiers encoded within barcodes like a “flag” that define: The nature of the data: what is it? The length of the data: how many digits/characters, or variable to what length? The format of the data: numeric/alpha-numeric, particular sequence (e.g., YYMMDD) GS1 GTIN Batch/Lot Number 2016 GS1 US All Rights Reserved 77

Application Identifier Overview The most common AIs include those required in the FDA DSCSA and UDI Rule: (01) GTIN (17) Expiration Date (10) Batch or Lot Number (21) Serial Number GS1 AI for GTIN GS1 AI for Expiry Date GS1 AI for Batch/Lot Number GS1 AI for Serial Number 2016 GS1 US All Rights Reserved 78
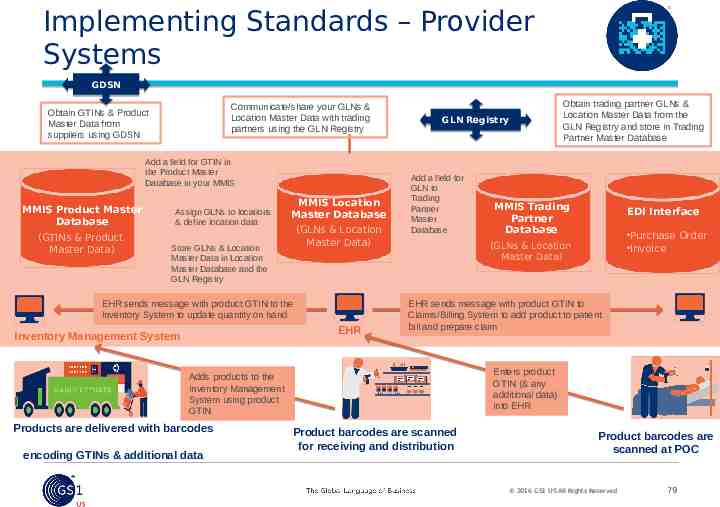
Implementing Standards – Provider Systems GDSN Communicate/share your GLNs & Location Master Data with trading partners using the GLN Registry Obtain GTINs & Product Master Data from suppliers using GDSN Add a field for GTIN in the Product Master Database in your MMIS MMIS Product Master Database (GTINs & Product Master Data) Assign GLNs to locations & define location data MMIS Location Master Database (GLNs & Location Master Data) Store GLNs & Location Master Data in Location Master Database and the GLN Registry EHR sends message with product GTIN to the Inventory System to update quantity on hand EHR Inventory Management System GLN Registry Add a field for GLN to Trading Partner Master Database encoding GTINs & additional data MMIS Trading Partner Database EDI Interface Purchase Order Invoice (GLNs & Location Master Data) EHR sends message with product GTIN to Claims/Billing System to add product to patient bill and prepare claim Enters product GTIN (& any additional data) into EHR Adds products to the Inventory Management System using product GTIN Products are delivered with barcodes Obtain trading partner GLNs & Location Master Data from the GLN Registry and store in Trading Partner Master Database Product barcodes are scanned for receiving and distribution Product barcodes are scanned at POC 2016 GS1 US All Rights Reserved 79

GTIN – BD example 00382903065073 Each Level GTIN 30382903065074 Shelf Pack GTIN 50382903065078 Case GTIN 2016 GS1 US All Rights Reserved 80

GTIN Implementation Steps from Use Cases Step 1: Establish Executive Support Step 2: Form a GTIN Implementation Team Step 3: Develop Project Communication Internal and External with suppliers Step 4: Assess Information Systems (ERP) Step 5: Identify/Obtain GTINs Select source (GDSN, GUDID, GPO, Supplier) Step 6: Engage Suppliers for Pilot & Testing Step 7: Conduct Transactional Testing Step 8: Create Standard Operating Procedures REPEAT ABOVE STEPS FOR NEXT SUPPLIER 2016 GS1 US All Rights Reserved 81

Global Data Synchronization Network (GDSN) 2016 GS1 US All Rights Reserved 82

Data Flow via GDSN The GS1 Global Registry is a single repository where basic data is registered. The GS1 Global Registry identifies the data pool location of source data. Data Pools provide data that is standards conformant, and interoperable in the GDSN . The data pool performs the transactions of sending and receiving validated product information between partners inside or outside the data pool. Select one data pool as a SINGLE point of entry to the GDSN Step 1 : Load Data Step 2 : Register Data Step 3 : Subscription Request Step 4 : Publish Data Step 5 : Recipient Confirmation 2016 GS1 US All Rights Reserved 83

GS1 Workgroups and Support Support supply chain standardization through adoption of GS1 data standards Provide GS1 Standards support in your implementations Collaborate with stakeholders in our community and connect the many colleagues standardizing and utilizing AIDC solutions 2016 GS1 US All Rights Reserved 84

Workgroup Activity at GS1 US Medical Devices Community Group Calls Price Accuracy Initiative GTINs in Practice (Beth Gibson [email protected]) Held every two weeks To join and receive meeting invitations, log into the GS1 Healthcare US Community room at http://community.gs1us.org/apps/org/workgroup/transactions action/ Healthcare Provider Advisory Group (Salil Joshi [email protected]) Held monthly To join and receive meeting invitations, log into the GS1 Healthcare US Community room at http://community.gs1us.org/apps/org/workgroup/gs1stds patcare/ Pharmaceutical Secure Supply Chain Group Call (Peter Sturtevant [email protected]) Held every other week To join, just log into the GS1 Healthcare US Community room at http://community.gs1us.org/apps/org/workgroup/rx securesc/ 2016 GS1 US All Rights Reserved 85

Next Steps Engage with your suppliers Contact your IT vendors (ERP, Clinical, EHR) and check functionality available in systems Develop Pilot Initiatives and Start Implementation Any Questions Please Feel Free To Contact me at: Salil Joshi [email protected] 609-620-4522 2016 GS1 US All Rights Reserved 86

Questions 2016 GS1 US All Rights Reserved 87

Contact Information Salil Joshi Senior Director, Industry Engagement – Hospital Providers GS1 US Corporate Headquarters Princeton Pike Corporate Center 1009 Lenox Drive, Suite 202 Lawrenceville, NJ 08648 USA T 609.620.4522 E [email protected] www.gs1us.org 2016 GS1 US All Rights Reserved 88






