Now let us consider some microstructural transformations
14 Slides179.00 KB

Now let us consider some microstructural transformations Recovery, Recrystallization & Grain Growth Part of MATERIALS SCIENCE Guide & AALearner’s Learner’s Guide ENGINEERING AN INTRODUCTORY E-BOOK Anandh Subramaniam & Kantesh Balani Materials Science and Engineering (MSE) Indian Institute of Technology, Kanpur- 208016 Email: [email protected], URL: home.iitk.ac.in/ anandh http://home.iitk.ac.in/ anandh/E-book.htm

We had noted the following in our introduction to phase transformations Phase Transformations Microstructural Transformations When one phase transforms to another phase it is called phase transformation. Often the word phase transition is used to describe transformations where there is no change in composition. In a phase transformation we could be concerned about phases defined based on: Structure e.g. cubic to tetragonal phase ess Property e.g. ferromagnetic to paramagnetic phase ase PPhhas re ture rucctu stru rost icro M Mic s orm rmaatitioonn raannssffo T T l a r u ra t c tu tru Micrroosstr s Phase transformations could be classified based on: ormatioonn sfo rannsf Tra es T PPhhaasse Kinetic: Mass transport Diffusional or Diffusionless Thermodynamic: Order (of the transformation) 1st order, 2nd order, higher order. Often subtler aspects are considered under the preview of transformations. E.g. (i) roughening transition of surfaces, (ii) coherent to semi-coherent transition of interfaces. Phase transformations are associated with change in one or more properties. Hence for microstructure dependent properties we would like to additionally ‘worry about’ ‘subtler’ transformations, which involve defect structure and stress state (apart from phases). Therefore the broader subject of interest is Microstructural Transformations. We now take up three microstructural transformations: Recovery, Recrystallization & Grain Growth

Cold Work Click here to know more about strengthening mechanisms We now introduce a ‘technical term’ called Cold Work. We will arrive at a formal definition of the term at the end of this topic. Cold work is an important method to increase the strength of metals (& alloys), especially for those, wherein other methods like precipitation hardening are not available. Notes. Cold work can be used to augment other strengthening mechanisms. Cold working is not a good strengthening mechanism for materials, wherein the service temperature is ‘high’. Cold work does not involve change in composition and hence has its benefits. Strengthening due to cold work may be a ‘by product’ of shaping of metals by deformation processing (like extrusion, forging, wire drawing, etc.) at ‘low temperatures’ For now, we use a ‘working definition’ of cold work as: Plastic deformation in the temperature range (0.3 – 0.5) Tm COLD WORK. We will refine this definition soon. During cold work the point defect density (vacancies, self interstitials ) and dislocation density increase. This leads to an increase in the internal energy of the material. Typical cold working techniques are rolling, forging, extrusion etc. Cold working is typically done on ductile metals and alloys (e.g. Al, Cu, Ni) and is a standard method of increasing the strength of soft metals like Aluminium.

Point defects and dislocations have strain energy associated with them. (1 -15) % of the energy expended in plastic deformation typically is stored in the form of strain energy (in these defects) The material becomes battery of energy! The amount of energy stored depends on the material, temperature, strain rate and type of deformation, grain size, etc. The cold worked material is in a microstructurally metastable state. Depending on the severity of the cold work the dislocation density can increase 4-6 orders of magnitude or more. The material becomes stronger, but less ductile. Cold work point defect density dislocation density Annealed material dislocation (106 109 ) Cold work Stronger material dislocation (1012 1014 )
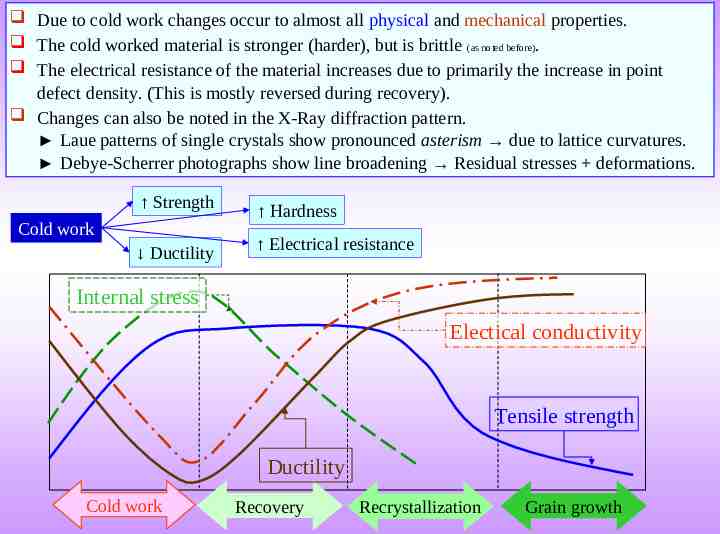
Due to cold work changes occur to almost all physical and mechanical properties. The cold worked material is stronger (harder), but is brittle (as noted before). The electrical resistance of the material increases due to primarily the increase in point defect density. (This is mostly reversed during recovery). Changes can also be noted in the X-Ray diffraction pattern. Laue patterns of single crystals show pronounced asterism due to lattice curvatures. Debye-Scherrer photographs show line broadening Residual stresses deformations. Strength Cold work Ductility Hardness Electrical resistance Internal stress Electical conductivity Tensile strength Ductility Cold work Recovery Recrystallization Grain growth
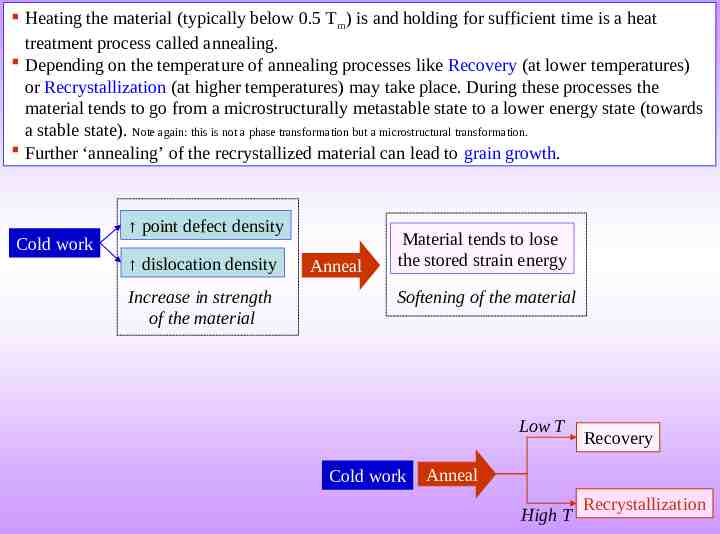
Heating the material (typically below 0.5 Tm) is and holding for sufficient time is a heat treatment process called annealing. Depending on the temperature of annealing processes like Recovery (at lower temperatures) or Recrystallization (at higher temperatures) may take place. During these processes the material tends to go from a microstructurally metastable state to a lower energy state (towards a stable state). Note again: this is not a phase transformation but a microstructural transformation. Further ‘annealing’ of the recrystallized material can lead to grain growth. Cold work point defect density dislocation density Increase in strength of the material Anneal Material tends to lose the stored strain energy Softening of the material Low T Cold work Recovery Anneal High T Recrystallization
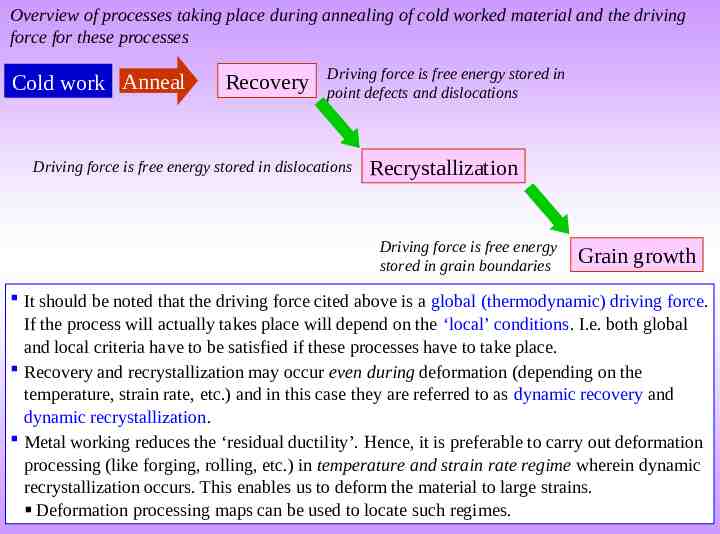
Overview of processes taking place during annealing of cold worked material and the driving force for these processes Cold work Anneal Recovery Driving force is free energy stored in point defects and dislocations Driving force is free energy stored in dislocations Recrystallization Driving force is free energy stored in grain boundaries Grain growth It should be noted that the driving force cited above is a global (thermodynamic) driving force. If the process will actually takes place will depend on the ‘local’ conditions. I.e. both global and local criteria have to be satisfied if these processes have to take place. Recovery and recrystallization may occur even during deformation (depending on the temperature, strain rate, etc.) and in this case they are referred to as dynamic recovery and dynamic recrystallization. Metal working reduces the ‘residual ductility’. Hence, it is preferable to carry out deformation processing (like forging, rolling, etc.) in temperature and strain rate regime wherein dynamic recrystallization occurs. This enables us to deform the material to large strains. Deformation processing maps can be used to locate such regimes.

Recovery Recovery takes place at low temperatures of annealing (after cold work). “Apparently there no change in microstructure” (i.e. if seen in an optical microscope, the microstructure looks similar before and after recovery). Two processes which occur during recovery are: Reduction in point defect density ( their reconfiguration), Annihilation of dislocations and their arrangement into low energy configurations. Note: not all point defects and dislocaitons participate in the above processes. It was noted that excess point defects are created during cold work. During recovery these are absorbed by processes which include (there are other processes which also may be active): at surface or grain boundaries or by dislocation climb process. During recovery, random dislocations (statistically stored dislocations) of opposite sign come together and annihilate each other. However, the overall reduction in the dislocation density by this process is small. Dislocations of same sign arrange into low energy configurations. Edge dislocations ‘rearrange’ to form Tilt boundaries Screw dislocations ‘rearrange’ to form Twist boundaries. The formation of low angle tilt and twist boundaries is termed as POLYGONIZATION (figure in next page). Hence, the overall reduction in dislocation density is small during recovery.
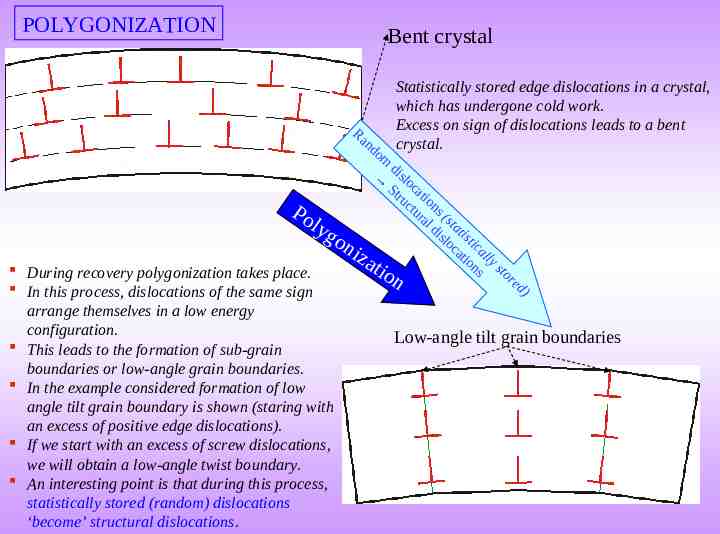
POLYGONIZATION Bent crystal Ra nd om Statistically stored edge dislocations in a crystal, which has undergone cold work. Excess on sign of dislocations leads to a bent crystal. d islo St cat r u io ctu ns Po r a (s l d ta lyg i sl t i st on oc i c at all iza io y n s st o tio During recovery polygonization takes place. r In this process, dislocations of the same sign arrange themselves in a low energy configuration. This leads to the formation of sub-grain boundaries or low-angle grain boundaries. In the example considered formation of low angle tilt grain boundary is shown (staring with an excess of positive edge dislocations). If we start with an excess of screw dislocations, we will obtain a low-angle twist boundary. An interesting point is that during this process, statistically stored (random) dislocations ‘become’ structural dislocations. n ed ) Low-angle tilt grain boundaries
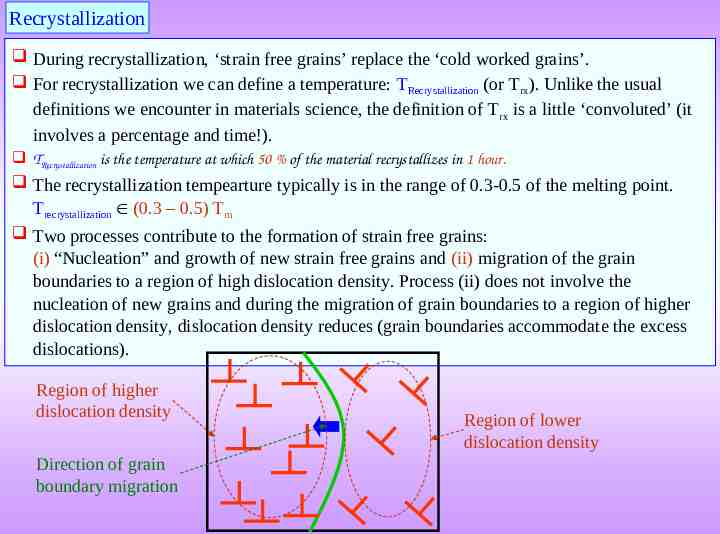
Recrystallization During recrystallization, ‘strain free grains’ replace the ‘cold worked grains’. For recrystallization we can define a temperature: TRecrystallization (or Trx). Unlike the usual definitions we encounter in materials science, the definition of T rx is a little ‘convoluted’ (it involves a percentage and time!). TRecrystallization is the temperature at which 50 % of the material recrystallizes in 1 hour. The recrystallization tempearture typically is in the range of 0.3-0.5 of the melting point. Trecrystallization (0.3 – 0.5) Tm Two processes contribute to the formation of strain free grains: (i) “Nucleation” and growth of new strain free grains and (ii) migration of the grain boundaries to a region of high dislocation density. Process (ii) does not involve the nucleation of new grains and during the migration of grain boundaries to a region of higher dislocation density, dislocation density reduces (grain boundaries accommodate the excess dislocations). Region of higher dislocation density Direction of grain boundary migration Region of lower dislocation density

Further points about recrystallization The driving force for recrystallization is the free energy difference between the deformed and undeformed material. G (recrystallization) G (deformed material) – G (undeformed material) Increased deformation (cold work) leads to a decrease in recrystallization temperature (T rx). If the initial grain size is smaller then the recrystallization temperature is lower. Higher amount of cold work low initial grain size leads to finer recrystallized grains. Higher temperature of working, lower strain energy stored, which will lead to a higher recrystallization temperature The rate of recrystallization is an exponential function of temperature. But, as the recrystallization process is a complex one (combination of many processes), the activation energy for recrystallization cannot be treated as a fundamental constant. The Trecrystallization is a strong function of the purity of the material. For very pure materials Trecrystallization is about 0.3 Tm [Trecrystallization (99.999% pure Al) 75oC ] For impure materials Trecrystallization (0.5 – 0.6) Tm [Trecrystallization (commercial purity) 275oC]. Impurity atoms tend to segregate to the grain boundary and retard their motion Solute drag (can be used to retain strength of materials at high temperatures). Second phase particles can also be used to pin down the grain boundary and impede its migration.
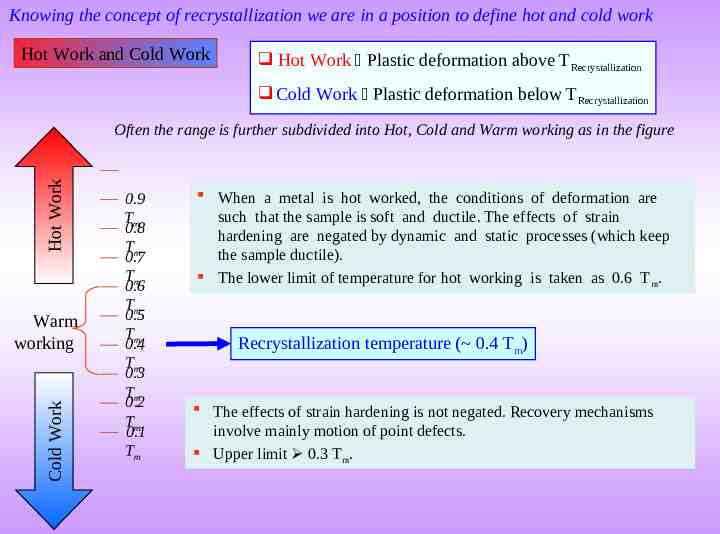
Knowing the concept of recrystallization we are in a position to define hot and cold work Hot Work and Cold Work Hot Work Plastic deformation above TRecrystallization Cold Work Plastic deformation below TRecrystallization Hot Work Often the range is further subdivided into Hot, Cold and Warm working as in the figure Cold Work Warm working 0.9 Tm 0.8 Tm 0.7 Tm 0.6 Tm 0.5 Tm 0.4 Tm 0.3 Tm 0.2 Tm 0.1 Tm When a metal is hot worked, the conditions of deformation are such that the sample is soft and ductile. The effects of strain hardening are negated by dynamic and static processes (which keep the sample ductile). The lower limit of temperature for hot working is taken as 0.6 T m. Recrystallization temperature ( 0.4 Tm) The effects of strain hardening is not negated. Recovery mechanisms involve mainly motion of point defects. Upper limit 0.3 Tm.

Grain growth The growth of larger grains at the expense of smaller ones, leading to the increase in the average grain size is termed as grain growth. (Obviously all the grains cannot grow!). This is also called ‘grain coarsening’. A related term to this is ‘Ostwald ripening’. Similar processes is observed in the case of precipitation, wherein larger precipitates grow at the expense of smaller ones, leading to an overall increase in the size of the precipitates (called precipitate coarsening). For grain growth to occur, both the global and the local criteria must be satisfied. The global criterion is easy to understand. Grain growth is Globally driven by reduction in grain boundary energy (per unit volume). The local condition is explained in the next page. If we make ‘hexagonal grains’ as in the figure below, the system will not coarsen. Grain growth will lead to a further drop in the strength of the material (i.e. after recrystallization has led to a considerable drop). The strength of a material depends on the grain size via the Hall-Petch relation (wherein larger grains imply a lower strength). A conceptual model of hexagonal grains, which will not coarsen, as the local criterion will not be satisfied.
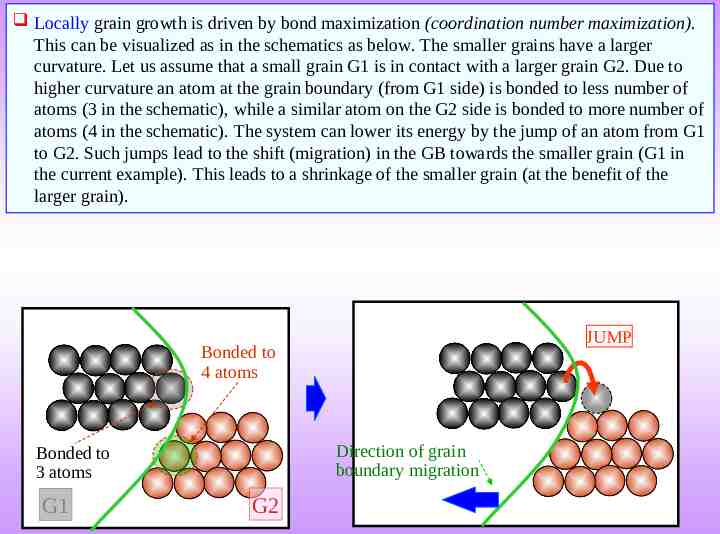
Locally grain growth is driven by bond maximization (coordination number maximization). This can be visualized as in the schematics as below. The smaller grains have a larger curvature. Let us assume that a small grain G1 is in contact with a larger grain G2. Due to higher curvature an atom at the grain boundary (from G1 side) is bonded to less number of atoms (3 in the schematic), while a similar atom on the G2 side is bonded to more number of atoms (4 in the schematic). The system can lower its energy by the jump of an atom from G1 to G2. Such jumps lead to the shift (migration) in the GB towards the smaller grain (G1 in the current example). This leads to a shrinkage of the smaller grain (at the benefit of the larger grain). JUMP Bonded to 4 atoms Direction of grain boundary migration Bonded to 3 atoms G1 G2






