NORTHWEST AIDS EDUCATION AND TRAINING CENTER Pre-exposure Prophylaxis
15 Slides990.55 KB

NORTHWEST AIDS EDUCATION AND TRAINING CENTER Pre-exposure Prophylaxis for HIV Prevention Efficacy and the importance of adherence Joanne Stekler, MD MPH August 20, 2015
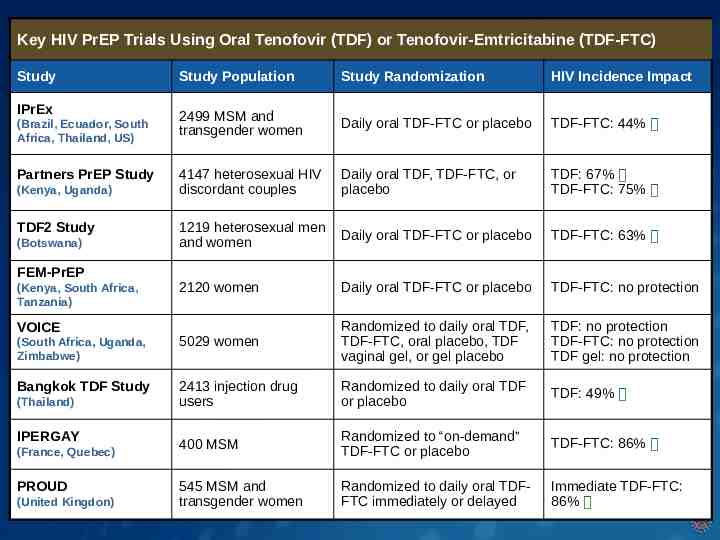
Key HIV PrEP Trials Using Oral Tenofovir (TDF) or Tenofovir-Emtricitabine (TDF-FTC) Study Study Population Study Randomization HIV Incidence Impact 2499 MSM and transgender women Daily oral TDF-FTC or placebo TDF-FTC: 44% 4147 heterosexual HIV discordant couples Daily oral TDF, TDF-FTC, or placebo TDF: 67% TDF-FTC: 75% 1219 heterosexual men and women Daily oral TDF-FTC or placebo TDF-FTC: 63% 2120 women Daily oral TDF-FTC or placebo TDF-FTC: no protection (South Africa, Uganda, Zimbabwe) 5029 women Randomized to daily oral TDF, TDF-FTC, oral placebo, TDF vaginal gel, or gel placebo TDF: no protection TDF-FTC: no protection TDF gel: no protection Bangkok TDF Study 2413 injection drug users Randomized to daily oral TDF or placebo TDF: 49% 400 MSM Randomized to “on-demand” TDF-FTC or placebo TDF-FTC: 86% 545 MSM and transgender women Randomized to daily oral TDFFTC immediately or delayed Immediate TDF-FTC: 86% IPrEx (Brazil, Ecuador, South Africa, Thailand, US) Partners PrEP Study (Kenya, Uganda) TDF2 Study (Botswana) FEM-PrEP (Kenya, South Africa, Tanzania) VOICE (Thailand) IPERGAY (France, Quebec) PROUD (United Kingdon)

Preexposure Prophylaxis (PrEP) for HIV Prevention in MSM iPrEx Trial: Methods Study Design - N 2499 HIV-seronegative men (or transgender women) - Sexual orientation: sex with men - All received risk reduction counseling, condoms, & STI Rx Regimens - Tenofovir-Emtricitabine (Truvada): 1 pill PO daily - Placebo: 1 pill PO daily Baseline HIV Infection - 10 subjects HIV-infected at time of enrollment Source: Grant RM, et al. N Engl J Med. 2010;363:2587-99.
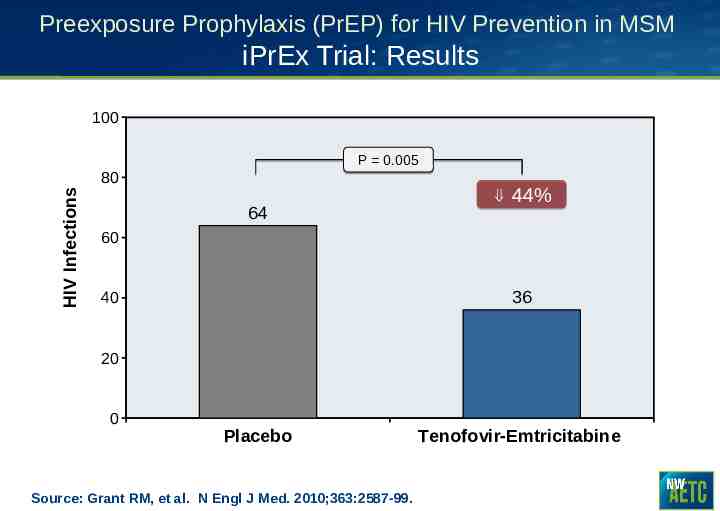
Preexposure Prophylaxis (PrEP) for HIV Prevention in MSM iPrEx Trial: Results 100 P 0.005 HIV Infections 80 64 44% 60 36 40 20 0 Placebo Source: Grant RM, et al. N Engl J Med. 2010;363:2587-99. Tenofovir-Emtricitabine
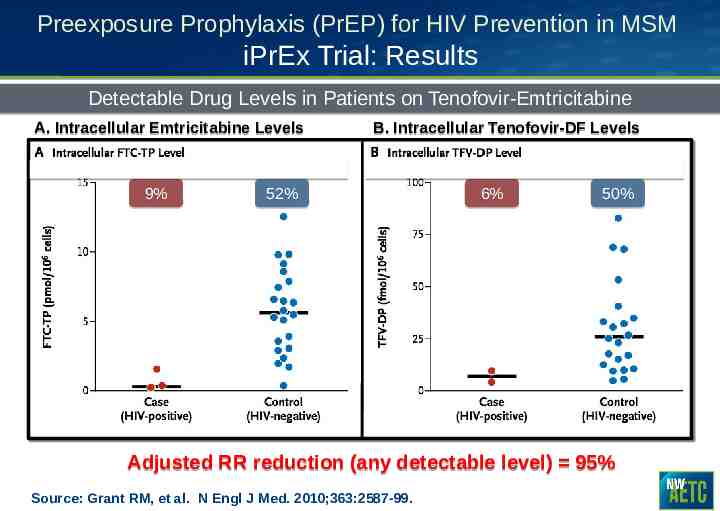
Preexposure Prophylaxis (PrEP) for HIV Prevention in MSM iPrEx Trial: Results Detectable Drug Levels in Patients on Tenofovir-Emtricitabine A. Intracellular Emtricitabine Levels 9% B. Intracellular Tenofovir-DF Levels 52% 6% 50% Adjusted RR reduction (any detectable level) 95% Source: Grant RM, et al. N Engl J Med. 2010;363:2587-99.

Vaginal and Oral Interventions to Control the Epidemic The VOICE Trial: Background VOICE Trial: Study Features N 5029 women Age 18-45 Setting: 14 sites in South Africa, Uganda, and Zimbabwe Eligibility: - Women who reported vaginal sex in previous 3 months - Not pregnant or breastfeeding - Willing to use effective contraception Regimens - Tenofovir 1% gel daily (TFV gel) - Tenofovir 300 mg po daily (TFV tablet) - Tenofovir 300 mg-emtricitabine 200 mg po daily (TDF-FTC tablet) Source: Marrazzo JM, et al. N Engl J Med. 2015;372:509-18.

Vaginal and Oral Interventions to Control the Epidemic VOICE Trial: Timeline Source: Marrazzo JM, et al. N Engl J Med. 2015;372:509-18.
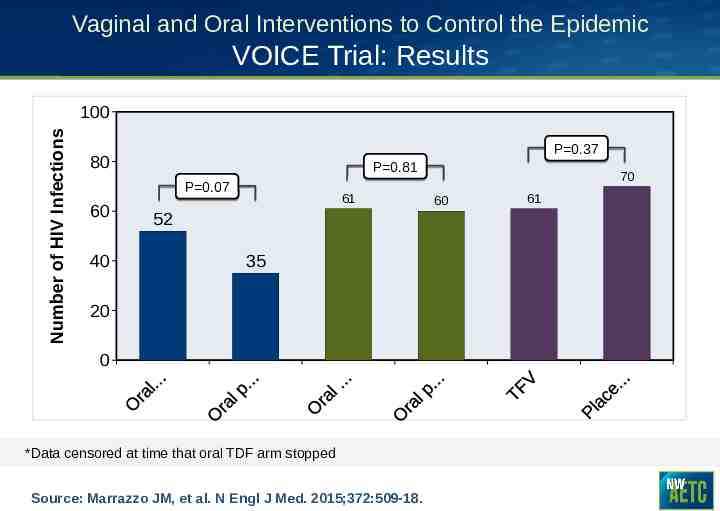
Vaginal and Oral Interventions to Control the Epidemic VOICE Trial: Results Number of HIV Infections 100 P 0.37 80 P 0.81 P 0.07 60 40 61 52 35 20 0 *Data censored at time that oral TDF arm stopped Source: Marrazzo JM, et al. N Engl J Med. 2015;372:509-18. 70 60 61
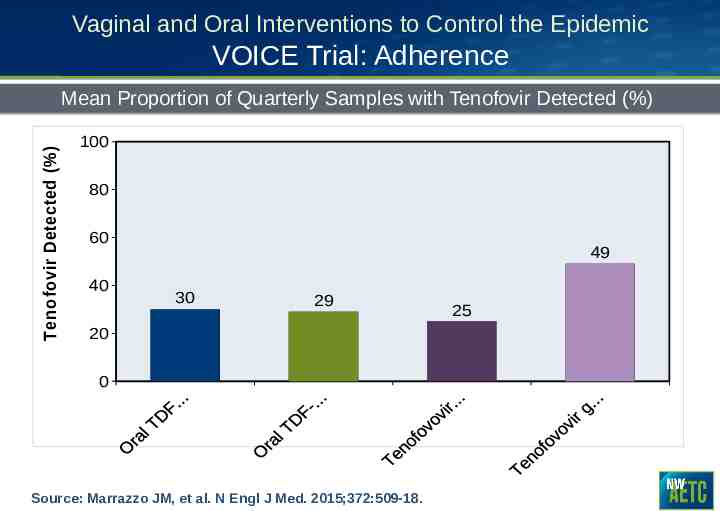
Vaginal and Oral Interventions to Control the Epidemic VOICE Trial: Adherence Tenofovir Detected (%) Mean Proportion of Quarterly Samples with Tenofovir Detected (%) 100 80 60 40 49 30 29 20 0 Source: Marrazzo JM, et al. N Engl J Med. 2015;372:509-18. 25
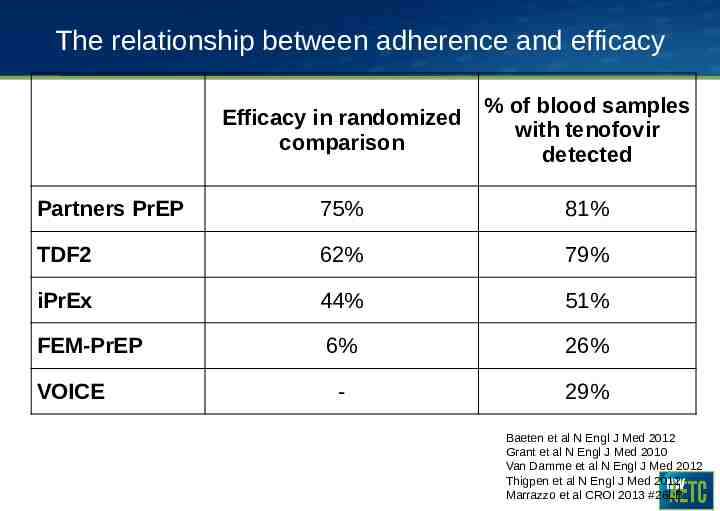
The relationship between adherence and efficacy Efficacy in randomized comparison % of blood samples with tenofovir detected Partners PrEP 75% 81% TDF2 62% 79% iPrEx 44% 51% FEM-PrEP 6% 26% - 29% VOICE Baeten et al N Engl J Med 2012 Grant et al N Engl J Med 2010 Van Damme et al N Engl J Med 2012 Thigpen et al N Engl J Med 2012 Marrazzo et al CROI 2013 #26LB
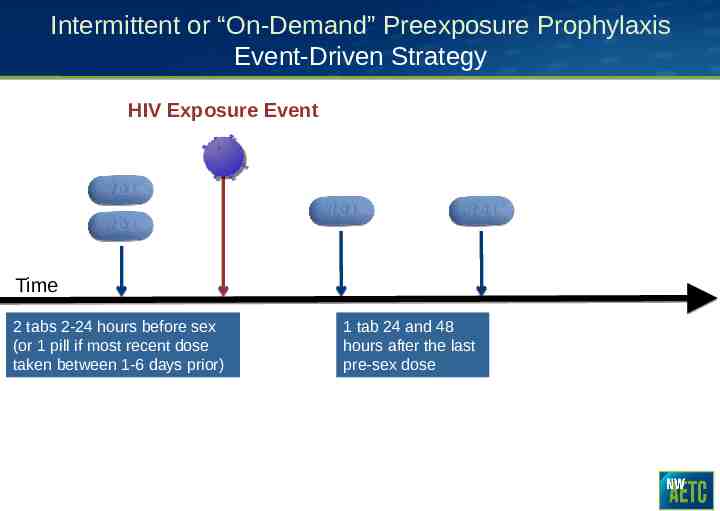
Intermittent or “On-Demand” Preexposure Prophylaxis Event-Driven Strategy HIV Exposure Event Time 2 tabs 2-24 hours before sex (or 1 pill if most recent dose taken between 1-6 days prior) 1 tab 24 and 48 hours after the last pre-sex dose

Intermittent or “On-Demand” PrEP for High-Risk MSM IPERGAY: Background Study Features N 400 high-risk men-who-have-sex-with-men (MSM) Setting: France and Canada Condomless anal sex with 2 partners in prior 6 months eGFR 60 mL/min All received risk-reduction counseling, condoms, and HAV and HBV vaccines if needed, as well as information about PEP Randomized to one of two arms Source: Molina JM, et al. CROI. 2015; Abstract 23LB.
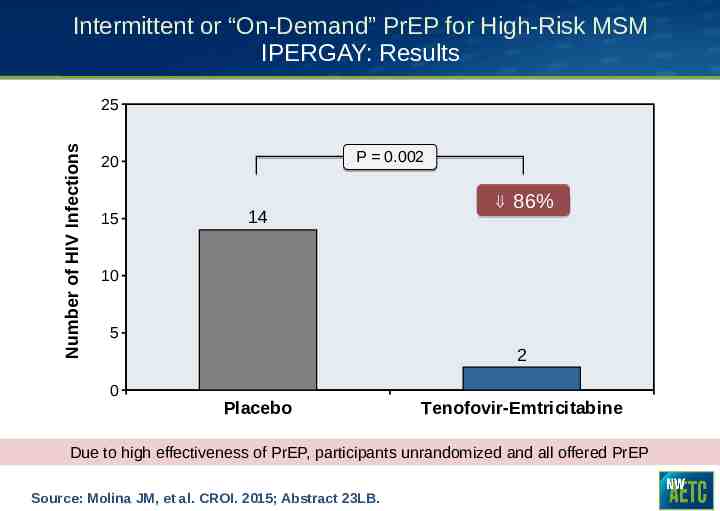
Intermittent or “On-Demand” PrEP for High-Risk MSM IPERGAY: Results Number of HIV Infections 25 P 0.002 20 15 14 86% 10 5 2 0 Placebo Tenofovir-Emtricitabine Due to high effectiveness of PrEP, participants unrandomized and all offered PrEP Source: Molina JM, et al. CROI. 2015; Abstract 23LB.
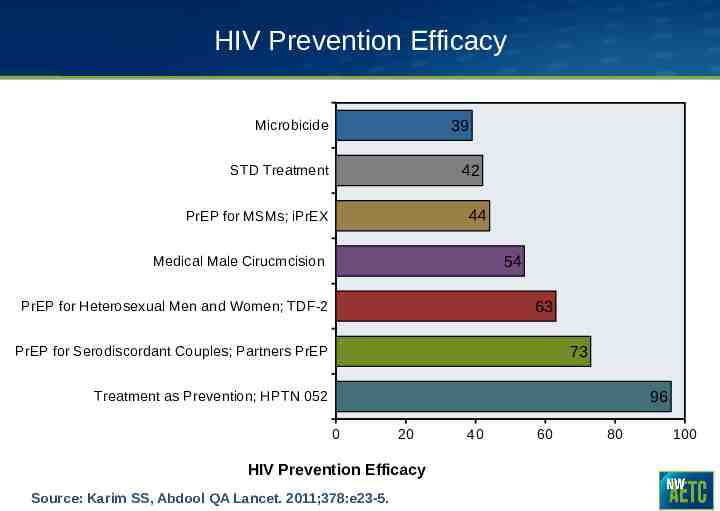
HIV Prevention Efficacy 39 Microbicide 42 STD Treatment 44 PrEP for MSMs; iPrEX 54 Medical Male Cirucmcision 63 PrEP for Heterosexual Men and Women; TDF-2 73 PrEP for Serodiscordant Couples; Partners PrEP 96 Treatment as Prevention; HPTN 052 0 20 HIV Prevention Efficacy Source: Karim SS, Abdool QA Lancet. 2011;378:e23-5. 40 60 80 100
Conclusion: “Highly active HIV prevention” HIV Testing & Serosorting? Condoms Needle Exchange HIV and STI PE P& Treatment PrE P Vaccines






