NIH Other Support & Biosketch Updates NIH Notice, NOT-OD-21-073,
47 Slides4.44 MB

NIH Other Support & Biosketch Updates NIH Notice, NOT-OD-21-073, contains policy points and includes links to other references that include further policy points. Effective May 25, 2021 – Other Support & Foreign Contracts Updates Report resources and/or financial support from all foreign and domestic entities, that are available to the researcher. Examples: laboratory personnel; high-value, rare materials (e.g., biologics, chemical, model systems, technology, etc Report supporting documentation, which includes copies (translated; machine-read ok) of contracts, grants or any other agreement specific to senior/key personnel foreign appointments and/or employment with a foreign institution for all foreign activities and resources providing consulting agreements when the PI will be conducting research through the consulting, In Kind Contributions: Materials (data, samples, etc.) from external collaborators: list source, summary description, and value (estimated ok) or estimated time commitment/effort PI/PDs need to sign their Other Support pages. Immediate notification of undisclosed Other Support. Of PI or other Senior/Key personnel on an active NIH grant Effective January 25, 2022 --New Biosketch requirements Personal Statement: include ongoing and completed research projects from the past three years that you want to draw attention to (previously known as research support) Positions, Scientific Appointments, and Honors: In reverse chronological order, include all positions and scientific appointments (domestic and foreign),including titled academic, professional, or institutional appointments, whether paid or unpaid, and whether fulltime, part-time, or voluntary/honorary NIH notice, Biosketch and Other Support templates posted on Research Operations website 1

Clinical Trial Office Going back to basics 2

CTO is moving beyond COVID focused research and going Back to Basics Prior to COVID, CTO was designing and implementing new customer service based initiatives CTO played an integral part in implementing the COVID research program at BMC Transitioned ACTIV Network trials and Community Engagement to Clinical Research Network COVID research is less time intensive, allowing CTO to return to process improvement projects Current initiatives being discussed today: 1. 2. 3. 4. Continue to encourage efficient communication across G&C and CTO for overlapping trials Improve communications on roles, responsibilities, and pre-award timeline expectation Centralize Ancillary Service tracking Create CTO live education: ClinCard, VelosCT/Epic, Other- looking for input 3

Back to Basics continued Additional initiatives: 5. Update departmental monthly meetings agenda – start April/May Trigger expense discussions Use new milestone tracker (summer) Review new Ancillary Services summary and forms 6. Implement pre-award status checklist / Create Velos Status reporting for study teams to be award of current pre-award tasks 7. Identify unique BU and BMC cross institutional clinical research areas for process improvement and develop solutions 8. Collaborate on urine pregnancy discussions 9. Aid in development of research encounter hospital and professional charge codes 10. Provide TriNetX support 11. Support VNA conversations to identify one partner for applicable clinical research protocols 12. Engage in pipeline conversations with sponsors 13. Incorporate community engagement costs in budget negotiations 14. Include research requirements in Infor implementation, SOPs, training, reporting 15. Create clear roles and responsibilities chart for CTO, department administrators, and study teams 4
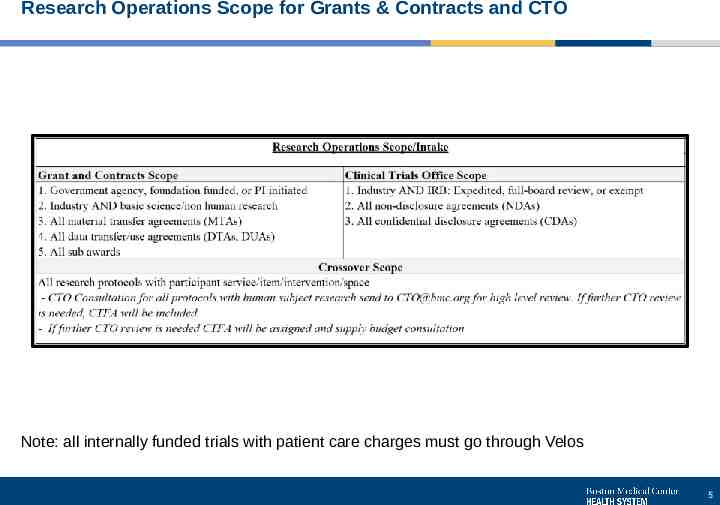
Research Operations Scope for Grants & Contracts and CTO Note: all internally funded trials with patient care charges must go through Velos 5
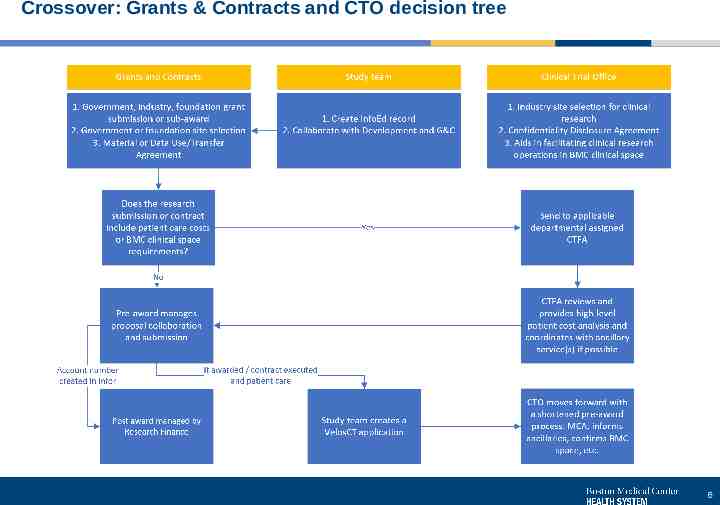
Crossover: Grants & Contracts and CTO decision tree 6
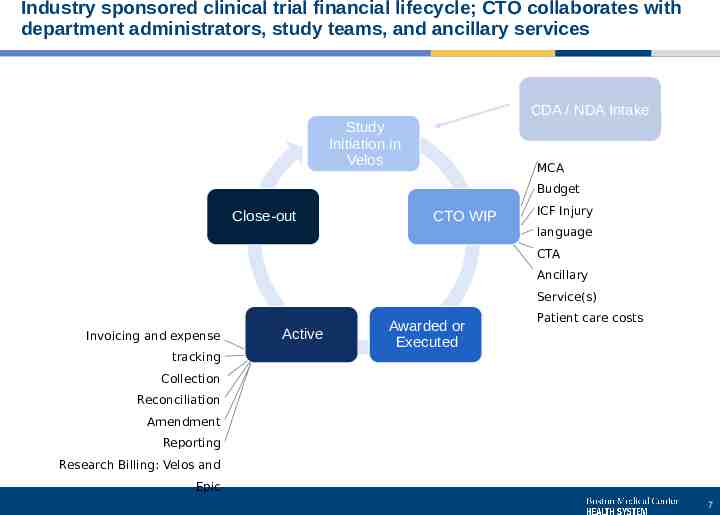
Industry sponsored clinical trial financial lifecycle; CTO collaborates with department administrators, study teams, and ancillary services CDA / NDA Intake Study Initiation in Velos MCA Budget Close-out CTO WIP ICF Injury language CTA Ancillary Service(s) Invoicing and expense tracking Active Awarded or Executed Patient care costs Collection Reconciliation Amendment Reporting Research Billing: Velos and Epic 7
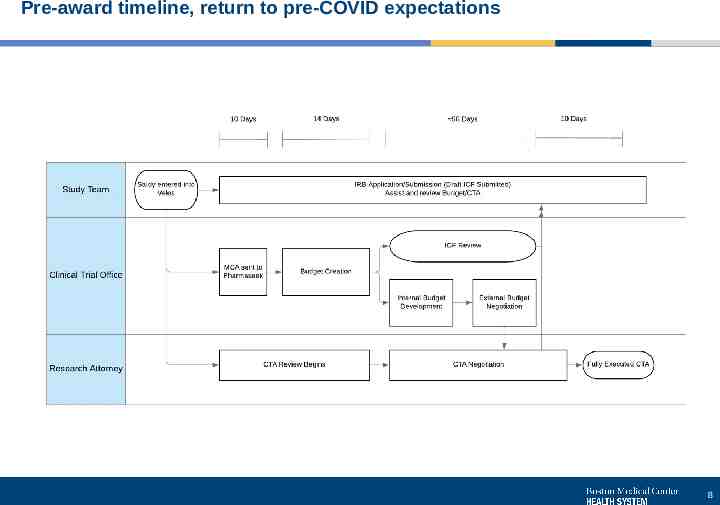
Pre-award timeline, return to pre-COVID expectations 8
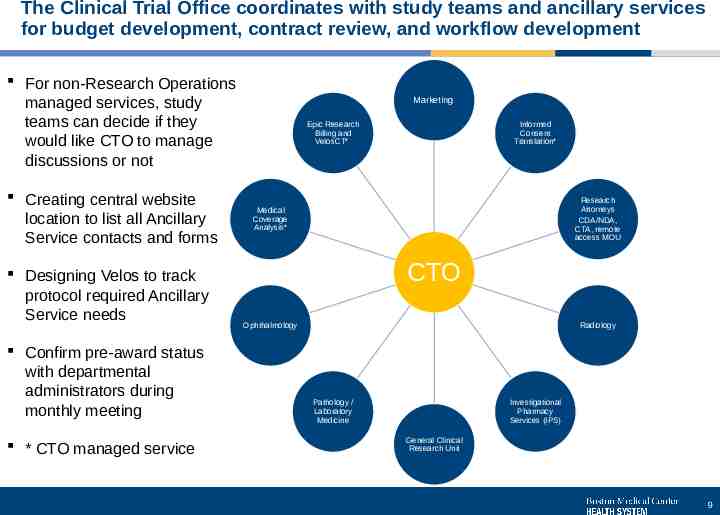
The Clinical Trial Office coordinates with study teams and ancillary services for budget development, contract review, and workflow development For non-Research Operations managed services, study teams can decide if they would like CTO to manage discussions or not Creating central website location to list all Ancillary Service contacts and forms Designing Velos to track protocol required Ancillary Service needs Confirm pre-award status with departmental administrators during monthly meeting * CTO managed service Marketing Epic Research Billing and VelosCT* Informed Consent Translation* Research Attorneys CDA/NDA, CTA, remote access MOU Medical Coverage Analysis* CTO Ophthalmology Radiology Pathology / Laboratory Medicine Investigational Pharmacy Services (IPS) General Clinical Research Unit 9

VelosCT/Epic, used for CTO intake AND Research Billing Compliance What studies are Required to Use VelosCT? All research that requires a service or intervention occurring within Boston Medical Center space, regardless of funding source All clinical research that is managed by the CTO, used for intake and may be used for research billing Some protocols may choose to use VelosCT to track participant enrollment but not used for research billing- this is optional What Is VelosCT and Why Is It Important? Connects financial, administrative and clinical research activities to help better manage research studies Helps to ensure compliant billing to Medicare and other 3rd party payers Enrolling/Associating Participants To VelosCT Calendar and Updating Visits Participants must be enrolled within 24 hours of the research visit, and associated to calendars with visits updated within 2448 hours of when they occur Failure to do so could result in the following billing compliance issues: Patients/insurance being incorrectly billed Additional communication to resolve issues Missing the timely filling limit Audits risk/findings Mistrust with our patients Confidentiality breech VelosCT Study Setup Overview Adding participants to the study Updating patient schedule 10
CTO Education Opportunities Hyperlinks or contact listed below: CTO website Velos and ClinCard education series will be held monthly Self administered VelosCT training MCA/Velos Determination Checklist CDA/NDA submission Remote Monitoring Workflow TriNetX support information- contact [email protected] Research re-start, remote monitoring recent communication Comments and questions welcome [email protected] 11

Clinical Research Network Overview April 12, 2021 12
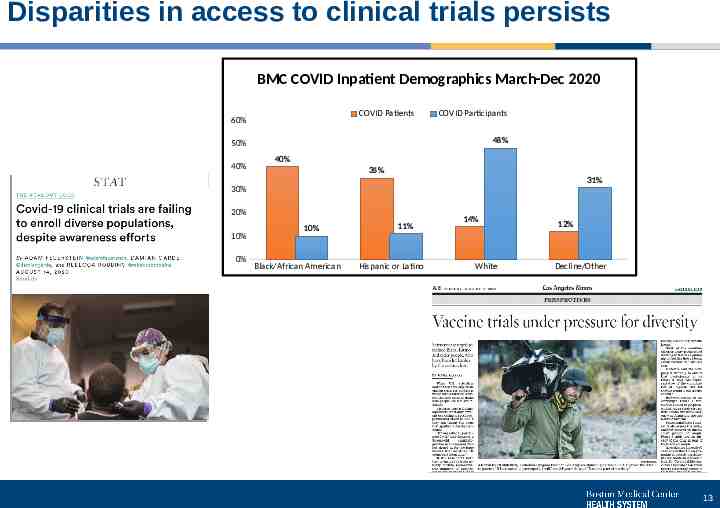
Disparities in access to clinical trials persists BMC COVID Inpatient Demographics March-Dec 2020 COVID Patients 60% COVID Participants 48% 50% 40% 40% 35% 31% 30% 20% 10% 0% 10% Black/African American 11% Hispanic or Latino 14% White 12% Decline/Other 13

New Research Infrastructure April ‘20Mar ’21: a multifaceted stakeholder approach to succeed COVID Scientific Review Committee Dedicated COVID scientific review committee to review feasibility, mission appropriateness & scientific merit Coordinated intake of COVID trials GCRU and BMC developed a COVID Inpatient and Ambulatory research coordination program GCRU is a critical partner in pre-award regulatory submissions and floor/clinic research activities Centralized Inpatient Research Facilitator to track: not approached, approached, declined, and enrolled New Research Biorepository Dedicated biorepository for the collection of research critical biospecimen samples Addition of Investigational Phlebotomy Services Protected research resources to support clinical trial blood collection Flagging of Research Patients in Epic Supported billing compliance and visibility of patients undergoing research to clinical teams Developed remote EMR access for monitors process ChartLink 14

New Research Infrastructure April ‘20Mar ’21: future of BMC clinical research is inclusive Identified immediate diversity and inclusion budget gaps across COVID trials Organized consent translation in 4 primary languages and collaboration with interpreter services Suggested transportation services in addition to participant stipend Collaborated with marketing and communications Hosted two interns from Urban College of Boston (UCB) in Michael Paasche-Orlow’s team Independent, non-profit 501(c)3 co-educational two-year college established to provide the opportunity for post-secondary education and professional advancement to those in the urban community traditionally underserved by higher education UCB enrolled student body is 82% Black or LantinX, 2/3 are learning English as a second language. Urban College grants an Associate of Arts degree as well as Certificate programs in 11 areas In 2020, Urban College launched a new program entitled The Research Apprenticeship Multicultural Partnership (RAMP) Program at Urban College of Boston. The RAMP Program leads to an academic certificate in Clinical Research Coordination and is aimed at training the next generation of clinical research professionals Created Clinical Research Network – March 2021 Determined by COVID-19 physicians and administration that ACTIV trials would become BMC’s priority Implemented dedicated team to oversee the NIH sponsored ACTIV studies, due to under resourced PIs Committed to improving diversity in research; engage BMC’s underserved population through ACTIV 15

COVID-19 Study Summary – Active/Completed Studies: 25 as of April 9, 2021 Research Focus Antiviral Antiviral Antiviral Antiviral C5 Inhibitor IL-1 Inhibitor IL-1b and IL-18 Inhibitor IL-6 Inhibitors IL-6 Inhibitors; Antiviral JAK Inhibitor Monoclonal Antibodies Monoclonal Antibody Testing Testing Testing Topoisomerase 2 Inhibitor Treatment Vaccine Total Description Favipiravir Hydroxychloroquine (Out PT TX) Hydroxychloroquine (Prophylaxis) Selinexor Eculizumab- EAP Canakinumab MAS825 Tocilizumab (TCZ) Tocilizumab & Remdesivir Ruxolitinib REGN10933 REGN10987 Anti-SPIKE Rapid Diagnostic Evaluation SIG-COVID STOP Etoposide Prone Positioning RNA Vaccine Completed Enrollment Studies (18) Principal Investigator Section Marathe, Jai Infectious Diseases Paasche-Orlow, Michael General Internal Medicine Paasche-Orlow, Michael General Internal Medicine Sanchorawala, Vaishali Hematology and Medical Oncology Ieong, Michael Pulmonary, Allergy, Sleep & Critical Care Medicine Neogi, Tuhina Rheumatology Siddiqi, Omar Pulmonary, Allergy, Sleep & Critical Care Medicine Lin, Nina Infectious Diseases Lin, Nina Infectious Diseases Shah, Bhavesh Hematology and Medical Oncology Paasche-Orlow, Michael General Internal Medicine Sagar, Manish Infectious Diseases Belok, Sam Pulmonary, Allergy, Sleep & Critical Care Medicine Kataria, Yachana Pathology & Laboratory Medicine Jacobson, Karen Infectious Diseases Sloan, Mark Hematology & Medical Oncology Bosch, Nicholas, Andrew Pulmonary, Allergy, Sleep & Critical Care Medicine Barnett, Elizabeth Infectious Diseases Research Focus Anticoagulant Anticoagulant and Antiplatelet C5 Inhibitor Immune Modulatory Agents RDV Sample Collection Testing Vaccine Total Description Enoxaparin Heparin or Enoxaparin Ravulizumab- RCT Infliximab/Abatacept/Cenicriviroc Biorepository Direct Antigen Rapid Test Pediatrics RNA Vaccine Studies Actively Recruiting (7) Principal Investigator Section Hamburg, Naomi Cardiovascular Medicine Ieong, Michael Pulmonary, Allergy, Sleep & Critical Care Medicine Ieong, Michael Pulmonary, Allergy, Sleep & Critical Care Medicine Linas, Benjamin Infectious Diseases Bhadelia, Nahid Infectious Diseases Kataria, Yachana Pathology Barnett, Elizabeth Infectious Diseases Enrollments Executed 5/18/2020 5/4/2020 5/4/2020 4/23/2020 6/8/2020 4/27/2020 6/10/2020 4/30/2020 6/28/2020 5/5/2020 10/23/2020 8/19/2020 5/12/2020 6/30/2020 6/25/2020 5/1/2020 4/20/2020 6/5/2020 4 17 62 20 15 23 10 26 9 0 11 20 12 1468 500 8 305 269 2510 Executed Est. Enrollment Enrollments 11/9/2020 10 5 9/15/2020 15 2 6/16/2020 20 4 11/3/2020 25 14 5/19/2020 700 89 4/28/2020 200 36 3/31/2021 11 9 981 159 16
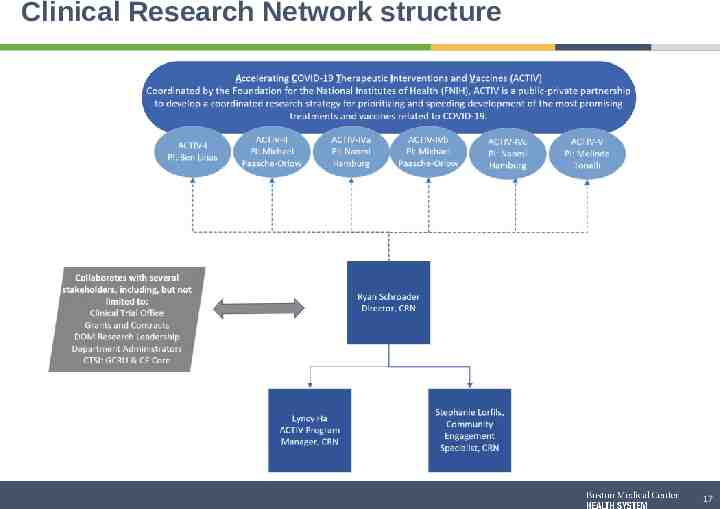
Clinical Research Network structure 17

Clinical Research Network Team Clinical Research Network (CRN) Mission: Develop a community engagement and equity support structure to ensure BMC is a world class research organization and is inclusive of our diverse patient population for the ACTIV trials. Scope of the CRN: Oversee lifecycle of the ACTIV COVID Research Trials Develop and deploy an ACTIV trial Community Engagement strategy with BMC care providers and patients Ryan Schroeder Lyncy Ha Stephaie Lorfils Director, Clinical Research Network ACTIV Program Manager Community Engagement Recruitment Specialist 18
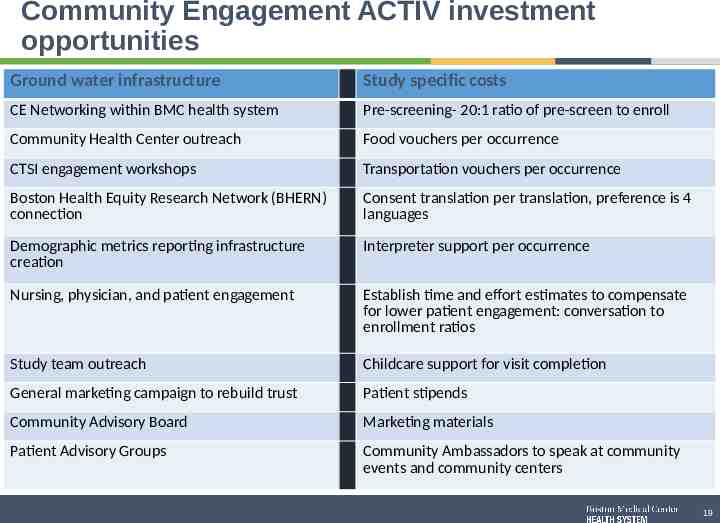
Community Engagement ACTIV investment opportunities Ground water infrastructure Study specific costs CE Networking within BMC health system Pre-screening- 20:1 ratio of pre-screen to enroll Community Health Center outreach Food vouchers per occurrence CTSI engagement workshops Transportation vouchers per occurrence Boston Health Equity Research Network (BHERN) connection Consent translation per translation, preference is 4 languages Demographic metrics reporting infrastructure creation Interpreter support per occurrence Nursing, physician, and patient engagement Establish time and effort estimates to compensate for lower patient engagement: conversation to enrollment ratios Study team outreach Childcare support for visit completion General marketing campaign to rebuild trust Patient stipends Community Advisory Board Marketing materials Patient Advisory Groups Community Ambassadors to speak at community events and community centers 19

Vision for the Future of Research at BMC BMC is a high performing site within leading edge research across all primary clinical areas Culture of research where physicians, nurses, staff and our community of patients understand and communicate the importance of diversity and inclusion in research BMC is a national leader and model institution where our intentional efforts to engage the community at every phase of research is reflected in the diversity of our clinical trial enrollment Our adoption and use of technology, and our role in establishing industry best practices demonstrates our commitment to innovation and reduces the burden of research participation for our patients 20

The NCDW (The NEW Clinical Data Warehouse) 21
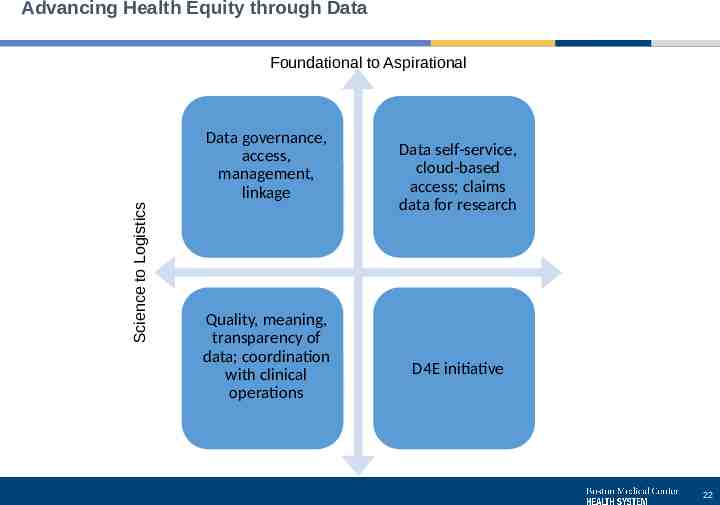
Advancing Health Equity through Data Science to Logistics Foundational to Aspirational Data governance, access, management, linkage Quality, meaning, transparency of data; coordination with clinical operations Data self-service, cloud-based access; claims data for research D4E initiative 22
Foundational Logistics: Efficient and Transparent Processes and Procedures Improved processes for requesting data and monitoring revenue ̶ Revised online interface captures information from data requestors efficiently, without redundancies to other systems ̶ Tracks incoming data requests to help CDW analysts work more efficiently Online billing and project tracking ̶ Allows CDW to project it’s yearly revenue and plan strategically ̶ Minimizes cost-shifting from departments to CDW Transparent Policies and Procedures ̶ Procedure manual Guidance on scope of CDW services Transparent logistic (e.g. data requesting, billing) and scientific procedures ̶ Assist data requestors with requests that translate into accurate data and reliable results ̶ Develop standard operating procedures to support data access, quality, reliability, confidentiality, and distribution Metrics Dashboard ̶ Visualization of metrics including wait times 23
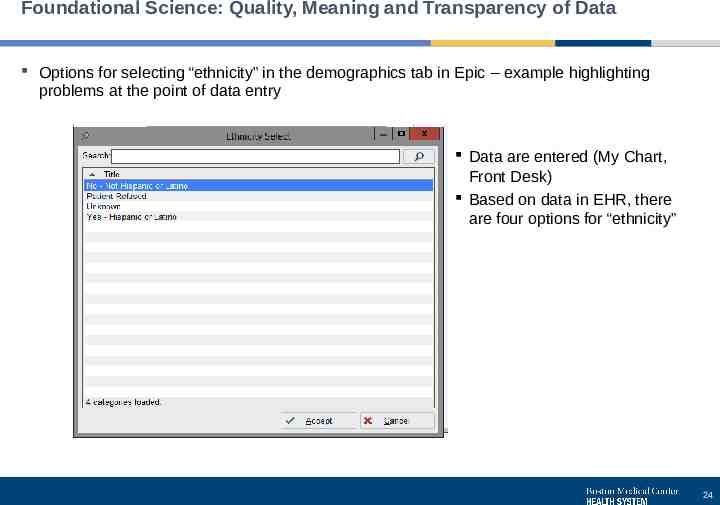
Foundational Science: Quality, Meaning and Transparency of Data Options for selecting “ethnicity” in the demographics tab in Epic – example highlighting problems at the point of data entry Data are entered (My Chart, Front Desk) Based on data in EHR, there are four options for “ethnicity” 24
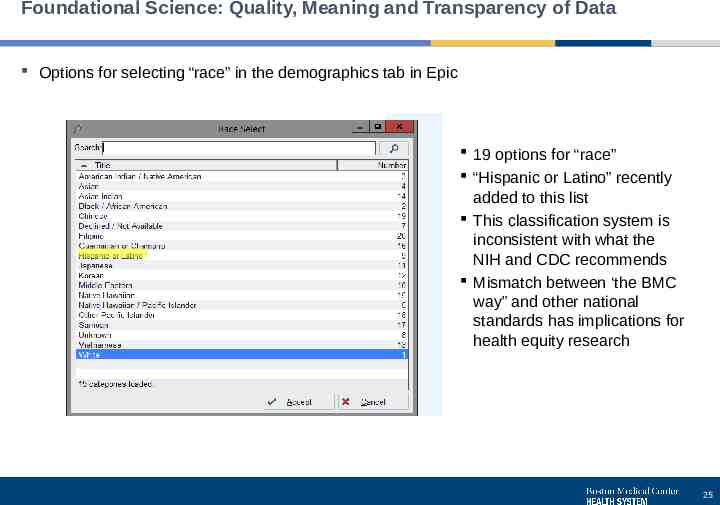
Foundational Science: Quality, Meaning and Transparency of Data Options for selecting “race” in the demographics tab in Epic 19 options for “race” “Hispanic or Latino” recently added to this list This classification system is inconsistent with what the NIH and CDC recommends Mismatch between ‘the BMC way” and other national standards has implications for health equity research 25

Foundational Science: Problems in classifying people by race or ethnicity Conflicting Information. Real patient examples from Epic (N 11,450): CDW team faces decisions about race/ethnicity data interpretation ̶ Example: in a CDW project addressing food insecurity screening and food pantry referrals among 14,000 patients, 30% have potentially conflicting race, ethnicity and ethnic origin data. ̶ Decision rules for interpretation of conflicting information How do we categorize people when BMC includes “Hispanic/Latino” as a race? Look at language preference, birth place, country of origin – and create an algorithm? ̶ Essential to work with clinical operations to reform the system beyond the CDW 26

Foundational Science: CDW Solutions Establish standardized workflows to ensure data quality, consistency, and efficiency ̶ Cultivate key partnerships across the medical campus to enhance understanding of nuances of data collection (e.g., pharmacy, clinical operations, patient registration, billing, THRIVE screening, food pantry, patient navigation, interpreter services, etc.) ̶ Development of standardized approaches to key health equity variables (e.g., race/ethnicity, THRIVE screening, identification of persons experiencing homelessness, language barriers/interpreter use, etc.) ̶ Development of BMC-tailored methods to identify special populations (e.g., identifying patients with substance use disorders through orders for Addiction Consults, prior OBAT visits, and inpatient orders for withdrawal monitoring in addition to ICD10 codes) ̶ Advisory Committee Rebecca Mishuris Allan Walkey Tuhina Neogi Jake Nudel Bill Adams 27

Aspirational Processes to Facilitate Research: data availability and access Expand the data available within the CDW ̶ Establish streamlined agreements with Community Health Centers to facilitate access to local data for health equity research ̶ Expand access to location-based social determinants of health data currently housed at BUSPH (e.g., geocoded addresses linked to neighborhood-based measures of opportunity or social vulnerability) ̶ CLAIMS DATA. CLAIMS DATA. CLAIMS DATA. Expand data access through use of the cloud-based TriNetX system for identification of local and national cohorts ̶ Trial Connect: supports invitations for collaboration with industry-sponsored clinical trials ̶ Query Builder: allows exploration of BMC data via self-service “drag-and-drop” interface ̶ Self-service analytics: tools for more advanced queries that compare outcomes and populations to support comparative effectiveness and health services research ̶ Limited dataset extracts: with special permission, researchers can download BMCspecific or national limited datasets for additional local analytics 28
Aspirational Science: Data for Equity (D4E) Initiative Goal: Advance health equity research in Boston by developing a de-identified, secure, highperformance data system that links clinical data from BMC and local CHCs with detailed data about where patients live Specifics ̶ Establish the BMC D4E data platform by converting our existing informatics framework into a more flexible model connected to a national research community ̶ Join the OHDSI (www.odhsi.org) national research community to leverage existing informatics tools, knowledge and resources ̶ Augment existing clinical data with additional data sources and linkages: BMC CDW: Geocode all addresses and link clinical data with BMC HealthNet claims D4E: Include census tract and zip code-level SDoH and air quality data ̶ Explore potential for D4E to link persons living in the same household ̶ Expand D4E to include Boston HealthNet CHCs ̶ Engage 2-3 pilot user groups within BMC/BU to submit specific project proposals and use the system for health equity research projects. ̶ Move local installation (server and workspace) into a HIPAA compliant cloud-based framework to support scalability and expanded research use 29

Infor FSM Financials and Supply Management Status Update April 2021

Background Refresher The Lawson system was end of life after 20 years and was re-implemented with the newly developed Infor system, on 10/1/20. This replaced the Finance, Supply Chain, Accounts Payable functions. A new Research module was implemented as well Several related systems had to be implemented along with Infor Chrome River – employee reimbursements MHC document management software Financial Data Warehouse This newer version is being improved and updated monthly on the 3rd Sat of the month automatically. The updates can be significant. Release notes are received 1.5 weeks prior to implementation and BMC reviews them for impact and notification as necessary. 31
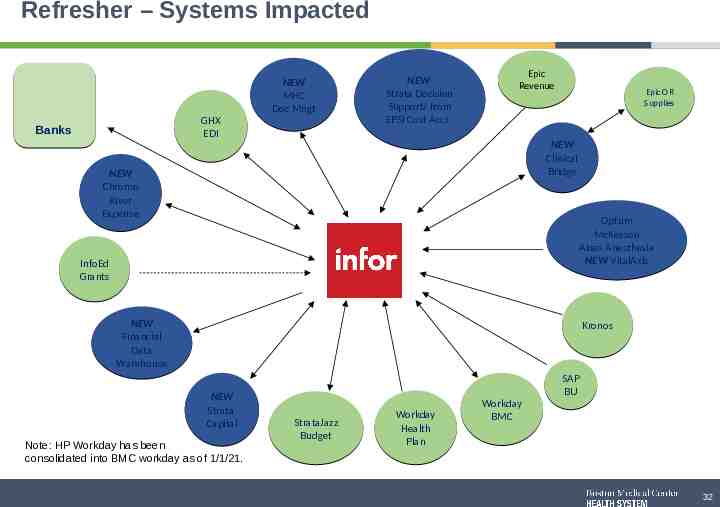
Refresher – Systems Impacted GHX EDI Banks NEW Strata Decision Support/ from EPSI Cost Acct. NEW MHC Doc Mngt Epic Revenue Epic OR Supplies NEW Clinical Bridge NEW Chrome River Expense Optum McKesson Abeo Anesthesia NEW VitalAxis Infor InfoEd Grants NEW Financial Data Warehouse Kronos NEW Strata Capital Note: HP Workday has been consolidated into BMC workday as of 1/1/21. StrataJazz Budget Workday Health Plan Workday BMC SAP BU 32

Post go-live: Prioritization of work and issues Ensuring back end processes that affect patient care work smoothly e.g. Requisitions getting processed and approved PO’s are issued and sent to vendors for procurement of goods Invoices moving through the steps to payment Access for users Ensuring users have access to the systems and information they need Month-end / Financial information Focus on data files as they move through processing Addressing any data issues that may become clear or arise e.g. mapping issues Focus on refinement of processes that impact the enterprise New on line forms, and information requirements are now being utilized With continued experience and evaluation, the need for modification will be done. A change management process for prioritization of ongoing work with governance, scoring, tracking of inventory, estimated level of effort is in the development process. 33

Infor Access Updates and Tips User access and reporting access Infor, Reporting and LDR access are all separate access and there are 2 different vendors Have been revisiting forms and provisioning with more experience FIS is working on process improvement of the existing forms based on feedback to: Combine the user access and reporting forms into a single form Make it more user friendly with guidance on how to complete it In process now – expect to update this in the next several weeks Dependencies for access and the steps to follow to assist you in obtaining access: 1. Need Workday ID and BMC email address to create Infor Access If necessary, complete the Visiting Personnel form to obtain a Workday ID from HRIS @ [email protected] 2. Active directory ticket through Service Now to create a network ID and BMC email address 3. Then create a ticket for Infor access and attach the completed user access form specifying the access you are requesting 34
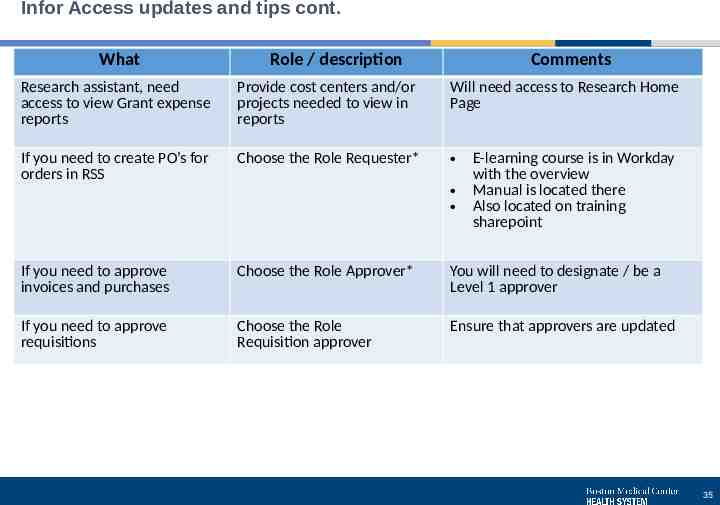
Infor Access updates and tips cont. What Role / description Comments Research assistant, need access to view Grant expense reports Provide cost centers and/or projects needed to view in reports Will need access to Research Home Page If you need to create PO’s for orders in RSS Choose the Role Requester* If you need to approve invoices and purchases Choose the Role Approver* You will need to designate / be a Level 1 approver If you need to approve requisitions Choose the Role Requisition approver Ensure that approvers are updated E-learning course is in Workday with the overview Manual is located there Also located on training sharepoint 35

Other Updates Open PO report Projects are in the system at the line level Currently no clean way in Infor to obtain a summary report by project of encumbrances Status: Some data clean up required completion and is now done Currently working with our reporting vendor to develop a report that will pull this information – logic under development Training/refreshers and updates High need training will be available either on Workday in e-learning format or in handout/manual format Requisitioning (e-learning w/manual posted), Chrome River (e Examples: learning), and others for reporting as well as tip sheets on training SharePoint Finalizing “keys to the car” items and updating them Major enhancements will be communicated and if part of these trainings will be updated Tip sheets will be provided for other changes and items that need to be communicated 36

Accounts Payable General Update Transition from manual, paper based process to invoice handling through three systems lead to inevitable learnings – these will smooth out and have allowed us to evaluate steps as issues come up, but this also created slowdowns at times FIS and Financial Operations meet regularly to review issues, including Correcting Approver roles fixed approvals going forward but earlier stuck approvals had to be addressed Regular metric tracking and review – in part to learn which metrics are most meaningful Alerting Supply Chain if any areas fall under them, such as older INR (invoice but not received) that needed to be cleaned up Focused temp staff on key areas to move larger than typical numbers of invoices out for approval Supply Chain has prioritized placing orders and ensuring the Hospital has supplies needed for patient care At times this has pulled them away from routine work that keep the requisition to receipt to invoice to payment process flowing smoothly They continue to be solid partners overall 37
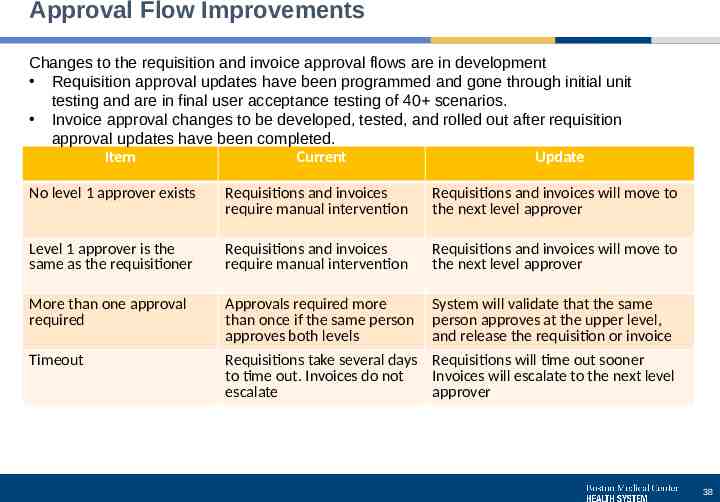
Approval Flow Improvements Changes to the requisition and invoice approval flows are in development Requisition approval updates have been programmed and gone through initial unit testing and are in final user acceptance testing of 40 scenarios. Invoice approval changes to be developed, tested, and rolled out after requisition approval updates have been completed. Item Current Update No level 1 approver exists Requisitions and invoices require manual intervention Requisitions and invoices will move to the next level approver Level 1 approver is the same as the requisitioner Requisitions and invoices require manual intervention Requisitions and invoices will move to the next level approver More than one approval required Approvals required more than once if the same person approves both levels Requisitions take several days to time out. Invoices do not escalate System will validate that the same person approves at the upper level, and release the requisition or invoice Requisitions will time out sooner Invoices will escalate to the next level approver Timeout 38

How you can help us Check AP’s pages on the Hub for current information Invoice handling process description Form for vendor adds, one-time payments, and recurring payments Tip sheets and link to Chrome River NEW tip sheet on running the Vendor Distribution report, which is available on the Infor Management Reports page Chrome River reimbursements through AP can be seen on the Vendor Distribution report An approval and report access listing and the change request form are available on the Infor Management Reports page Keep approvers for your areas updated – submit changes whenever someone starts, gets promoted, transfers or leaves Review approvers on the listing periodically (quarterly) Invoice handling Request new vendors before sending an invoice whenever possible If invoices come to you, forward to BMCHS [email protected] as soon as possible. Invoices must be approved before they can be paid, so there will be time to review the invoice after it’s sent for approval 39
How you can help us cont. Utilize the resources available on line that can assist Training SharePoint: http://share.bmc.org/InforAndRelatedFinancialSystemsTraining/default.aspx Service Now Tickets If you have issues, please submit a ticket so that these can be tracked, addressed and resolved. 40

Appendix
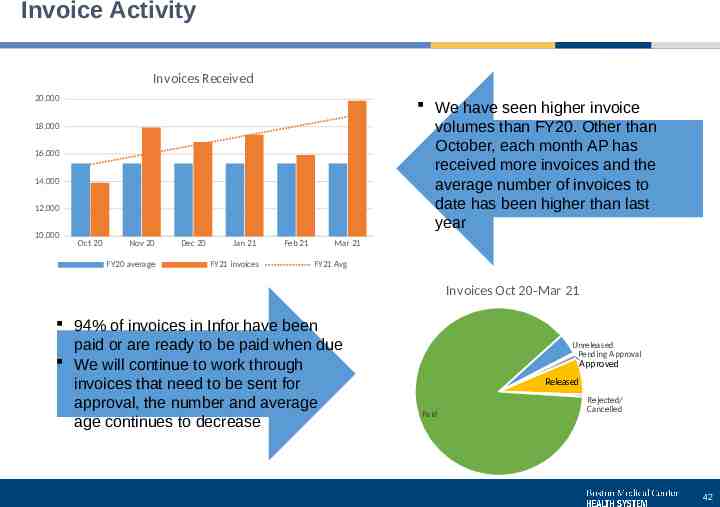
Invoice Activity Invoices Received 20,000 We have seen higher invoice volumes than FY20. Other than October, each month AP has received more invoices and the average number of invoices to date has been higher than last year 18,000 16,000 14,000 12,000 10,000 Oct 20 Nov 20 FY20 average Dec 20 Jan 21 FY21 invoices Feb 21 Mar 21 FY21 Avg Invoices Oct 20-Mar 21 94% of invoices in Infor have been paid or are ready to be paid when due We will continue to work through invoices that need to be sent for approval, the number and average age continues to decrease Unreleased Pending Approval Approved Released Paid Rejected/ Cancelled 42
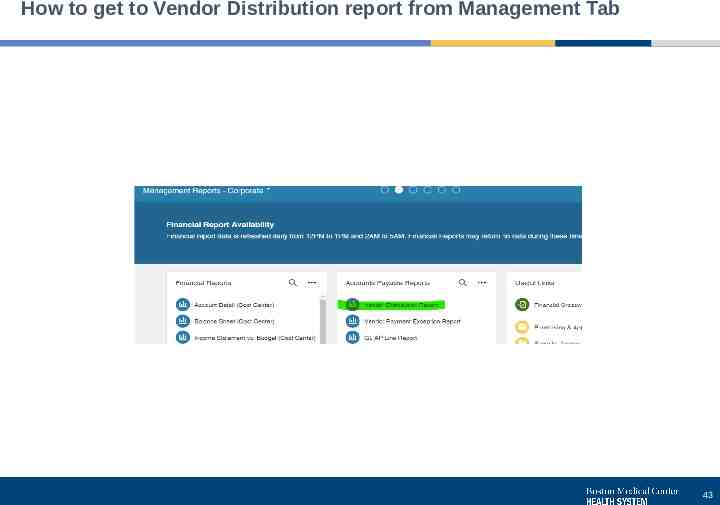
How to get to Vendor Distribution report from Management Tab 43
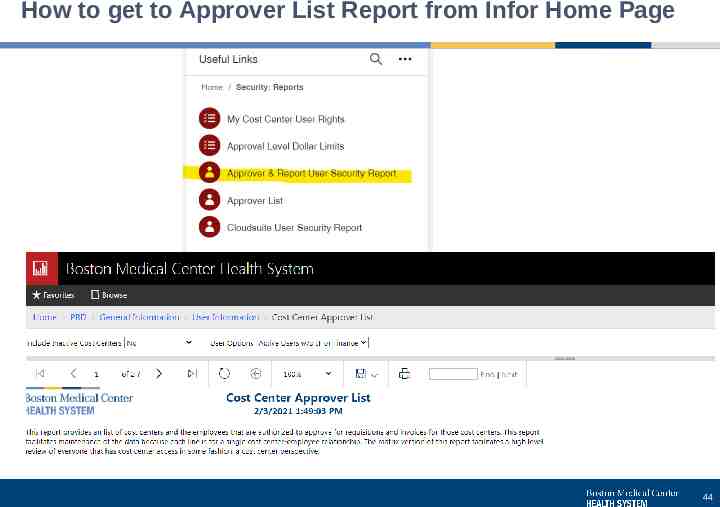
How to get to Approver List Report from Infor Home Page 44
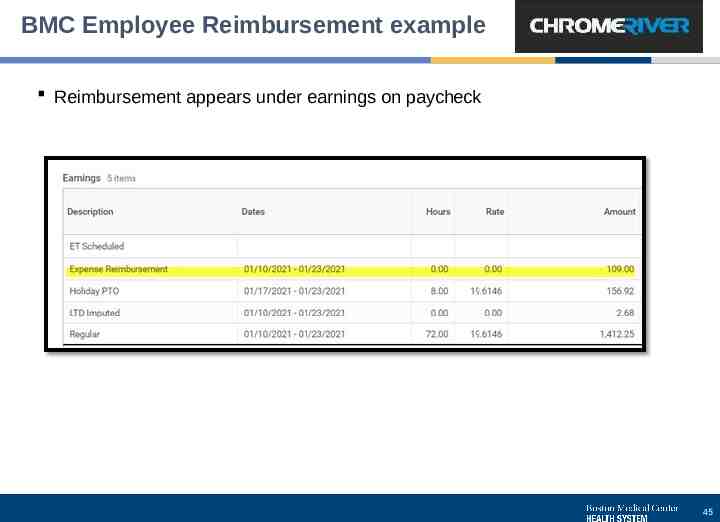
BMC Employee Reimbursement example Reimbursement appears under earnings on paycheck 45
Training SharePoint 46

Open Discussion and Questions Thank-you for attending today’s meeting!