Micelles to the Rescue: How Soap Transports Debris Ryan
20 Slides2.08 MB

Micelles to the Rescue: How Soap Transports Debris Ryan Kiddey Research Experience for Teachers (RET) The University of Akron

History of Soap Making - A Brief Overview Soap may be the first chemical compound ever synthesized by man! 2,800 B.C. – excavations reveal Babylonians were making soap by boiling animal fat with ashes. Used to clean wool and cotton used in textile making Used for medicinal purposes 1550 B.C. – Egyptian writing reveals soap Egyptians made soap by mixing animal and vegetable oils with alkaline (base) salts Egyptians medical scrolls recommend a soap for skin conditions
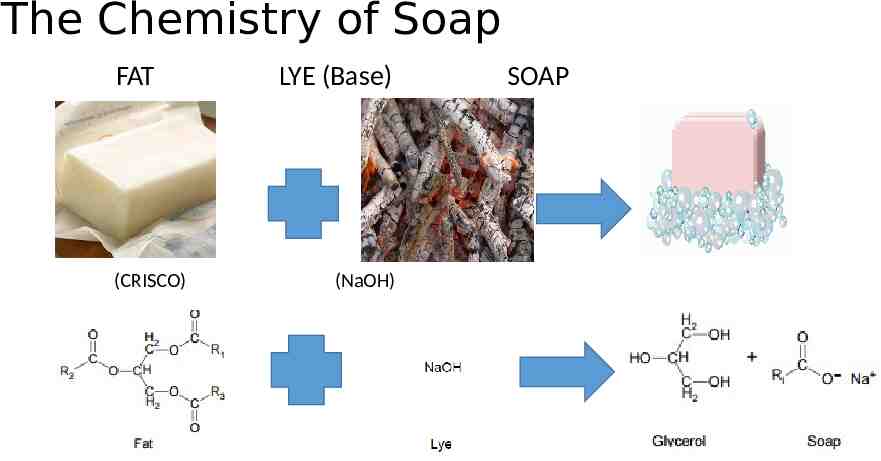
The Chemistry of Soap FAT (CRISCO) LYE (Base) (NaOH) SOAP
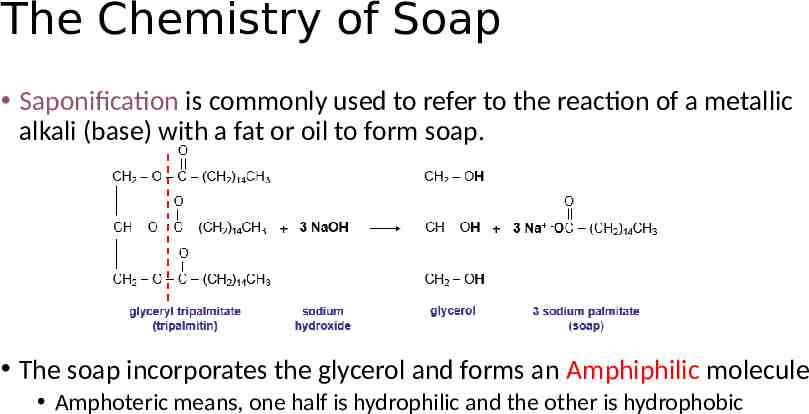
The Chemistry of Soap Saponification is commonly used to refer to the reaction of a metallic alkali (base) with a fat or oil to form soap. The soap incorporates the glycerol and forms an Amphiphilic molecule Amphoteric means, one half is hydrophilic and the other is hydrophobic
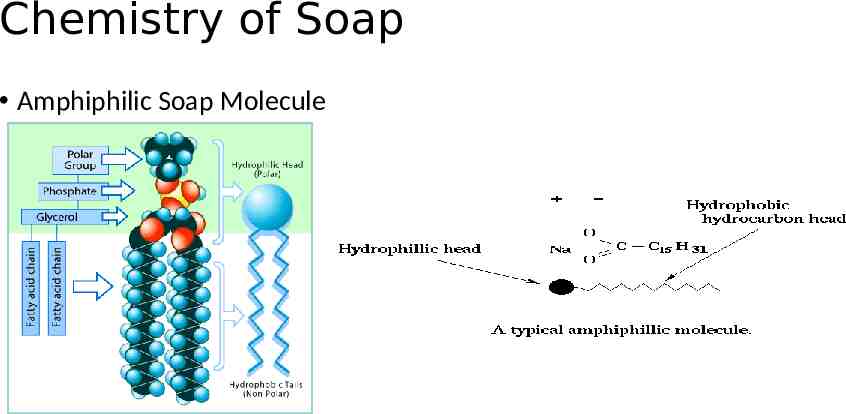
Chemistry of Soap Amphiphilic Soap Molecule

Chemistry of Soap Soap molecules act like a diplomat, improving the relationship between water and oil. How? When soap is added to the water, the hydrophilic (polar) heads of its molecules stay in the water (they are attracted to water), The long hydrophobic (nonpolar) chains join the oil particles and remain inwards (escaping from the water). This behavior forms a micelle.

Micelles Formation How Soap Works – Video When enough soap is added to water the soap molecules (sodium stearate) aggregate into micelles. Micelles are colloid particles Colloid particles stay suspended in solution due to collisions with other molecules in the solution The specific colloid formed is called an Emulsion Emulsion: when the dispersing solute (micelles) is in liquid form within a liquid solvent (water) When viewed under a microscope, colloids are very jittery due to Brownian Motion

Micelle Formation The “colloidal particles” are micelles which form immediately when the soap dissolves in water. This forms a ‘haze’ Individual micelles are too small to be seen by the naked eye. 50-100 nm in size

Micelle Formation You can tell if a substance is a colloid if the substance refracts light when shined into a colloidal solution. This refraction is called the Tyndall Effect.

Micelles form when using Soap Why can’t water just clean everything? Why use soap? Surface tension keeps water from easily spreading across a surface Surface tension must be weakened in order to get water to spread across an oily surface. Water is not attracted to oil because oil is a nonpolar substance Soap can weaken surface tension because soap is a surfactant Surfactant: SURface ACTive agents (they reduce surface tension of water) Surfactants inhibit the intermolecular attractions between water molecules, and this IMF attraction is what causes strong surface tension in water. The soap allows the water to flow more smoothly which allow micelles spread across an oily surface to collect grime to be washed away.

Soap Breaks Surface Tension Soap Breaking Surface Tension Marangoni Effect – Soap Powered Boat

Micelles to the rescue The micelles carry the oily dirt particles away from the skin (or surface). The water molecules hold the micelles in solution and carry them down the drain!
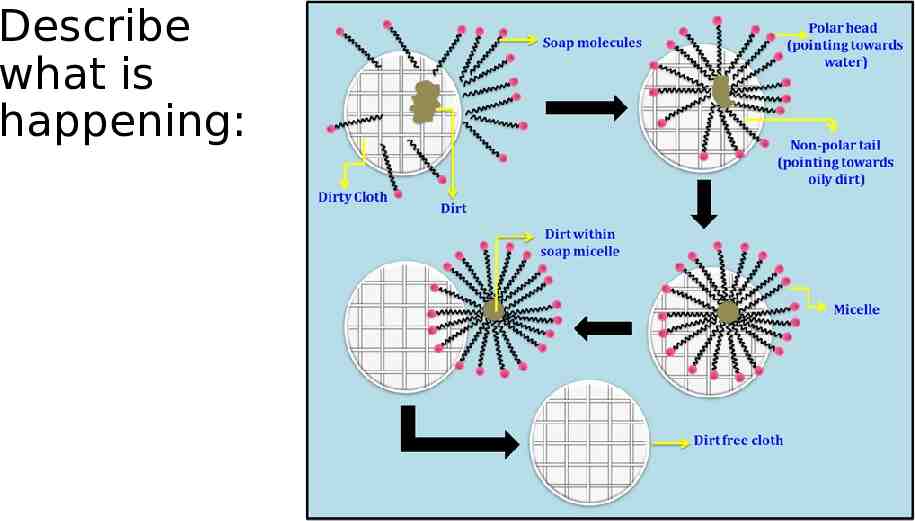
Describe what is happening:

Water Hardness Affects Micelles Formation What is water hardness? Water that has a lot of dissolved ions in it is considered hard water. A common dissolved ionic compound in water is Calcium Carbonate (CaCO3) 0-60 mg/L CaCO3 (Soft Water) 61-120 mg/L CaCO (Moderately Hard) 121-180 mg/L CaCO (Hard Water) 3 3 How does hard water affect soap? The hydrophilic (polar) end of an individual soap molecule is negatively charged. So what do you think happens when using soap in hard water?

Water Hardness Affects Micelle Formation (IN WATER) CaCO (s) Ca2 (aq) CO 2 (aq) 3 3 Micelles will not form because the soap molecule will no long be amphoteric, it will become non-polar “Soap Scum” will form and the soap will not clean properly.

Soap Scum: Calcium Ions attached to Soap Molecules
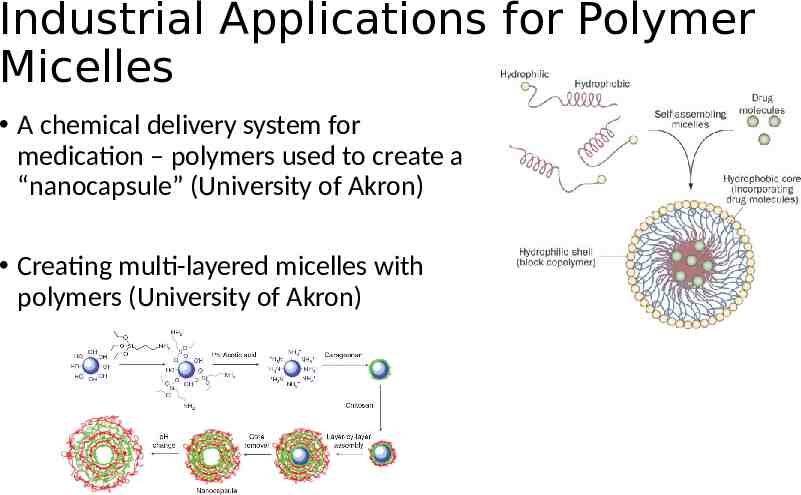
Industrial Applications for Polymer Micelles A chemical delivery system for medication – polymers used to create a “nanocapsule” (University of Akron) Creating multi-layered micelles with polymers (University of Akron)

Industrial Applications for Polymer Micelles Many lotions and beauty products contain chemicals that enhance the effect of micelles. Lubrizol Corporation makes chemical additives that are placed into lotions which enhance the effectiveness of micelles. Detergent companies have been researching better methods of using micelles for over 100 years.
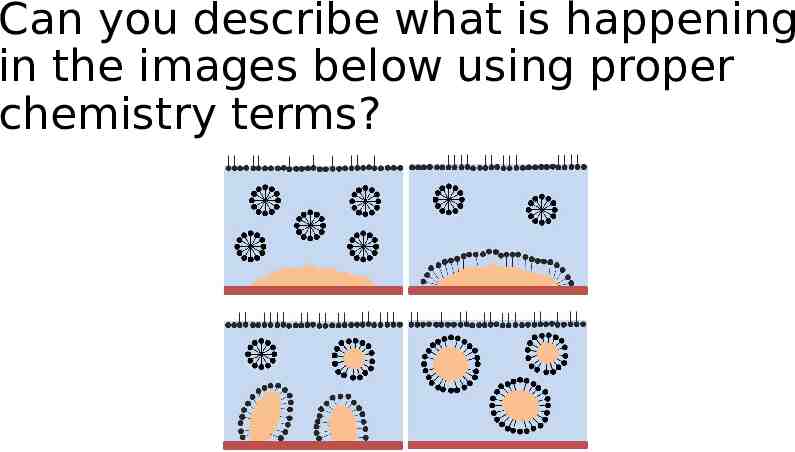
Can you describe what is happening in the images below using proper chemistry terms?
Reference http://www.soaphistory.net/ http://www.bioteach.ubc.ca/Bio-industry/Inex/ https://science360.gov/obj/tkn-video/81074969-11e0-4a2e-b674-8fc 8886fd9c3 Copyright 2007 Pearson Benjamin Cummings. All rights reserved. https://water.usgs.gov/edu/hardness.html Chemistry, A Molecular Approach Tro 2014 Pearson http://amrita.olabs.edu.in/?sub 73&brch 3&sim 120&cnt 1






