Just to review before we start… What is the melting point of
18 Slides692.00 KB
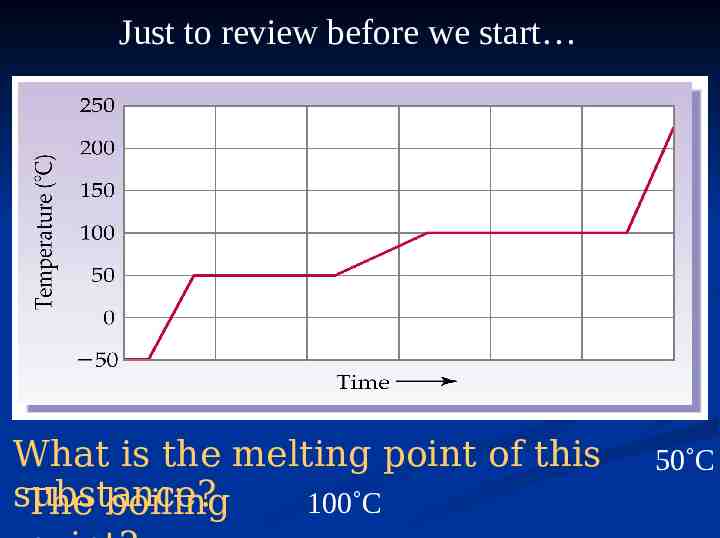
Just to review before we start What is the melting point of this substance? 100 C The boiling 50 C

Topic: Calculating Energy Changes at Phase Changes (Hv and Hf)
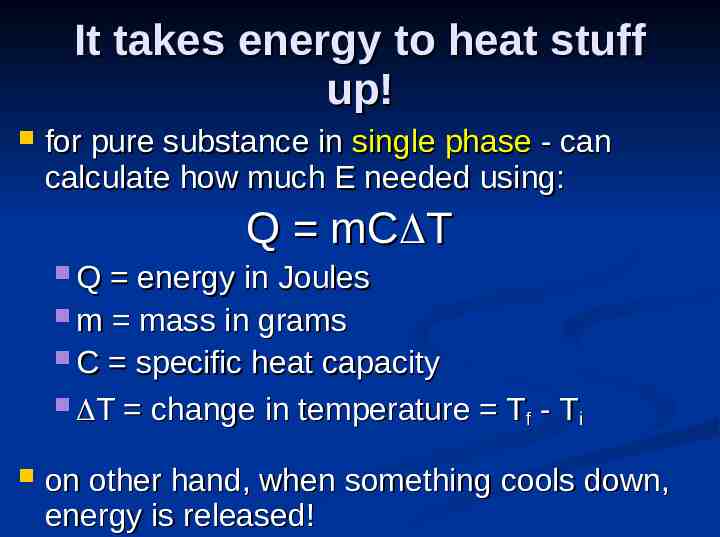
It takes energy to heat stuff up! for pure substance in single phase - can calculate how much E needed using: Q mC T Q energy in Joules m mass in grams C specific heat capacity T change in temperature T - T f i on other hand, when something cools down, energy is released!
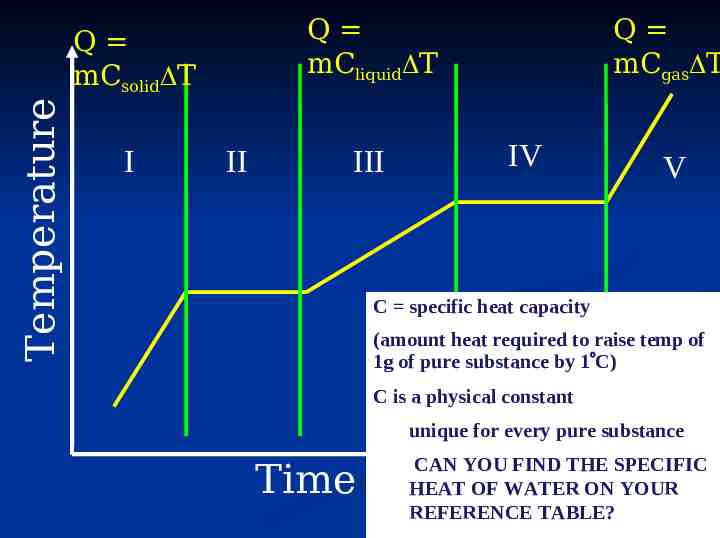
Q mCliquid T Temperature Q mCsolid T I II III Q mCgas T IV V C specific heat capacity (amount heat required to raise temp of 1g of pure substance by 1 C) C is a physical constant unique for every pure substance Time CAN YOU FIND THE SPECIFIC HEAT OF WATER ON YOUR REFERENCE TABLE?
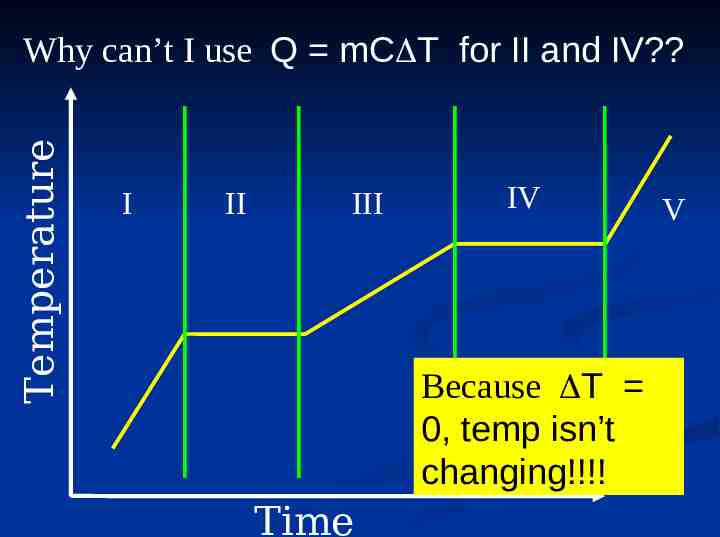
Temperature Why can’t I use Q mC T for II and IV? I II III IV Because T 0, temp isn’t changing!!!! Time V

So, how do we calculate the amount of energy required during a phase change? HF Heat of Fusion (Q mHF) HV Heat of Vaporization (Q mHV) We use one of these two constants instead of specific heat and delta T Q mC T
Hf Heat of Fusion is amount energy required to change 1 gram of pure substance from solid to liquid at its MP (meaning you aren’t changing the temperature) Is a physical constant Check out Reference Table B, what is the heat of fusion for water? The Equation Q mHf

How much heat is absorbed when 10 grams of ice melts at 0oC? Heat absorbed mass of substance x heat of fusion of substance Q mHf (10 g)(334 J/g) 3340 J Where does this energy go? Particles must overcome forces of attraction to move farther apart during phase change (s l)

HV Heat of Vaporization is the amount energy required to convert 1 gram of pure substance from liquid to gas at its BP (meaning you aren’t changing the temperature) Is a physical constant Check out Reference Table B, what is the heat of vaporization for water? The Equation Q mHv
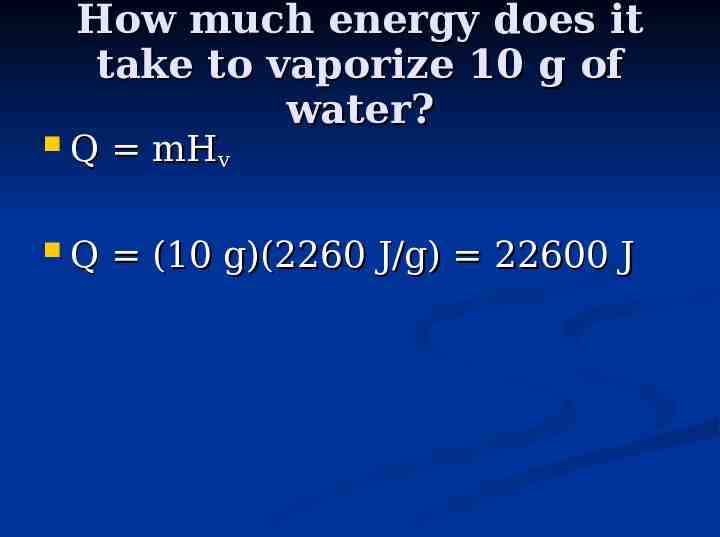
How much energy does it take to vaporize 10 g of water? Q mHv Q (10 g)(2260 J/g) 22600 J
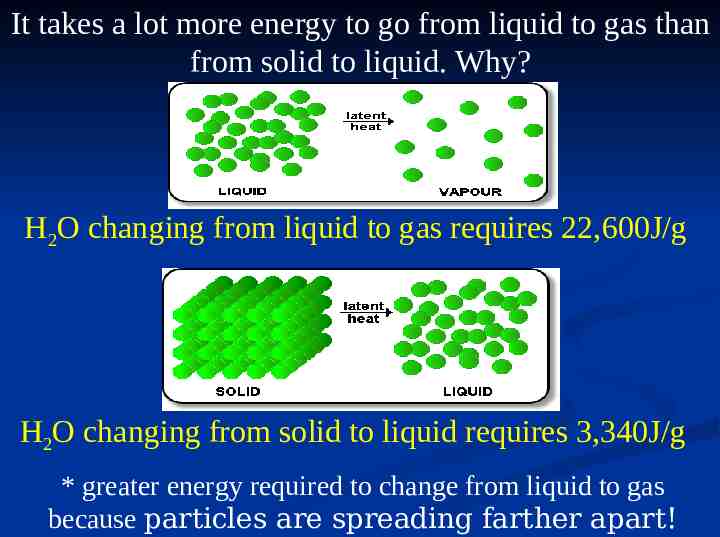
It takes a lot more energy to go from liquid to gas than from solid to liquid. Why? H2O changing from liquid to gas requires 22,600J/g H2O changing from solid to liquid requires 3,340J/g * greater energy required to change from liquid to gas because particles are spreading farther apart!
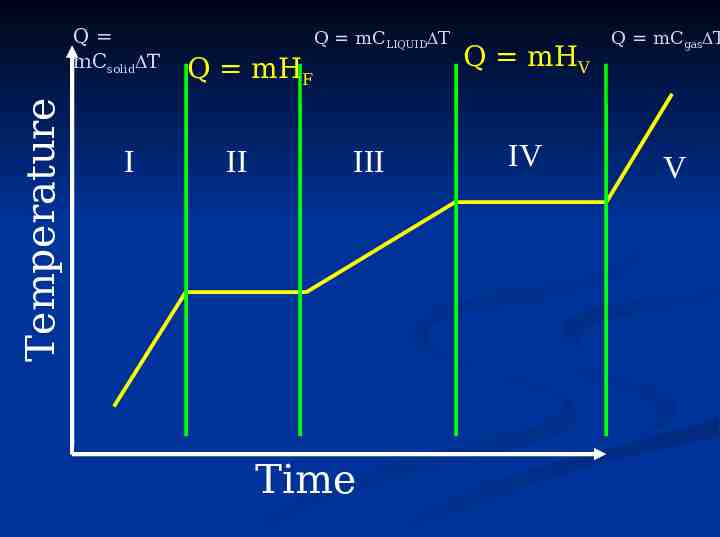
Temperature Q mCsolid T I Q mCLIQUID T Q mHF II III Time Q mHV IV Q mCgas T V
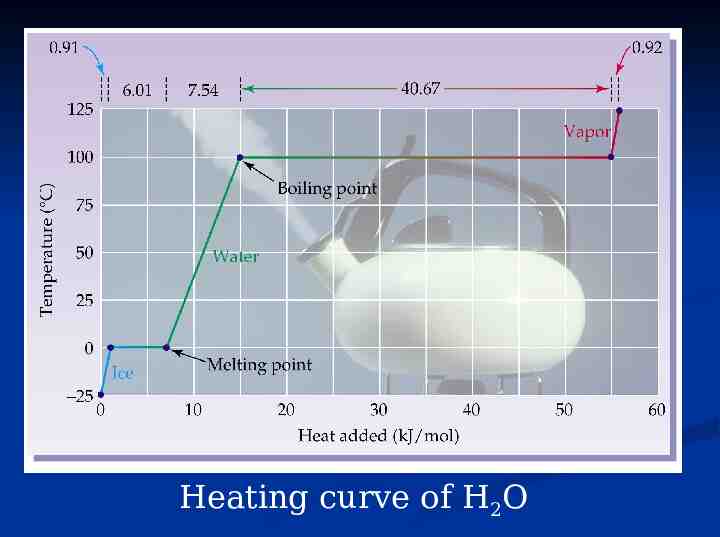
Heating curve of H2O

3 equations for Q 1. Q mC T 2. Q mHf 3. Q mHv figure out which to use depends on section of heating curve look for hints in word problem
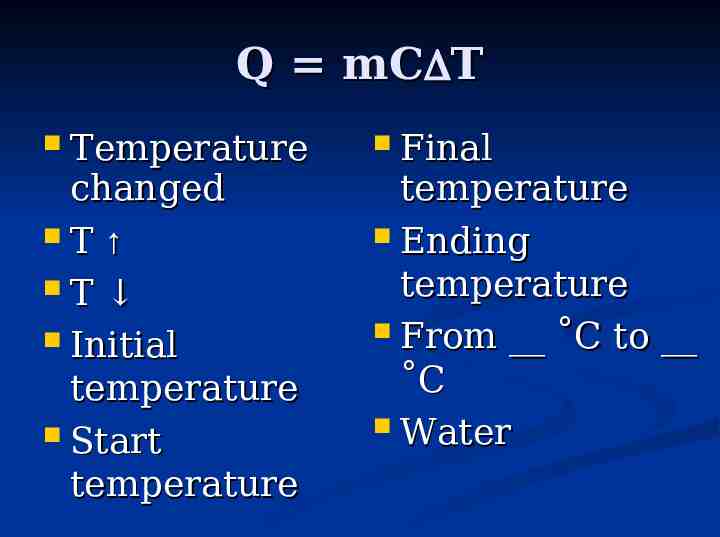
Q mC T Temperature changed T T Initial temperature Start temperature Final temperature Ending temperature From C to C Water

Q mHf Ice Freezing Melting Occurs at 0 C (for H2O) At constant temperature

Q mHv Steam Boiling Condensation Occurs at 100 C (for H2O) At constant temperature
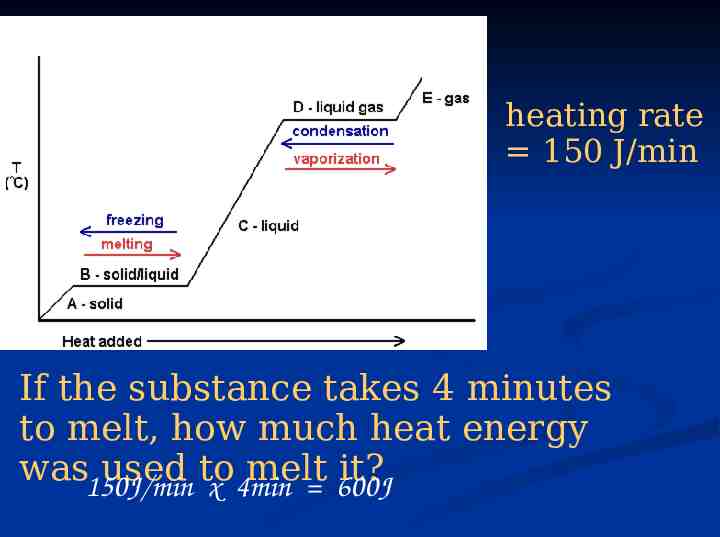
heating rate 150 J/min If the substance takes 4 minutes to melt, how much heat energy was150J/min used to melt it? x 4min 600J






