ENDOPLASMIC RETICULUM (ER Flagellum Rough ER Nuclear envelope
56 Slides2.42 MB
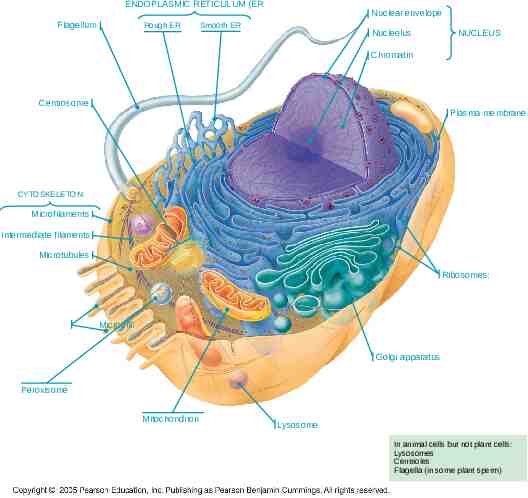
ENDOPLASMIC RETICULUM (ER Flagellum Rough ER Nuclear envelope Smooth ER Nucleolus NUCLEUS Chromatin Centrosome Plasma membrane CYTOSKELETON Microfilaments Intermediate filaments Microtubules Ribosomes: Microvilli Golgi apparatus Peroxisome Mitochondrion Lysosome In animal cells but not plant cells: Lysosomes Centrioles Flagella (in some plant sperm)

Centrifugation & Quantification Cellular Structure Centrifugation Beer-Lambert Equation Absorption Efficiency

Cells In a Leaf

Energy-Related Organelles: Mitochondria Bounded by double membrane – Cristae – Infoldings of inner membrane that encloses matrix – Matrix – Inner semifluid containing respiratory enzymes Involved in cellular respiration Produce most of ATP utilized by the cell 4

Energy-Related Organelles: Chloroplast Bounded by double membrane Inner membrane infolded – Forms disc-like thylakoids, which are stacked to form grana – Suspended in semi-fluid stroma Green due to chlorophyll – Green photosynthetic pigment – Found ONLY in inner membranes of chloroplast 5

Captures light energy to drive cellular machinery Photosynthesis – Synthesizes carbohydrates from CO2 & H2O – Makes own food using CO2 as only carbon source – Energy-poor compounds converted to enery rich compounds Chloroplast structure includes: 6 – Thylakoids, membranous sacs, stacked to form a granum – Stroma, the internal fluid

Purification of Cell Parts Understanding the roles of each cell component depends on methods to break (lyse) cells and separate cell components for analysis Cell lysis is accomplished by various techniques: blender, sonication, tissue homogenizer, hypotonic solution Separation of cell components generally involves centrifugation

Cell Fractionation Cell fractionation takes cells apart and separates the major organelles from one another Ultracentrifuges fractionate cells into their component parts Cell fractionation enables scientists to determine the functions of organelles Biochemistry and cytology help correlate cell function with structure

Forces Resulting from Spinning

Centrifugation A centrifuge is used to separate particles or even macromolecules: - Cells - Sub-cellular components - Proteins - Nucleic acids Basis of separation: - Size - Shape - Density Methodology: - Utilizes density difference between the particles/macromolecules and the medium in which these are dispersed - Dispersed systems are subjected to artificially induced gravitational fields

Homogenization Tissue cells Homogenate Differential centrifugation

Fixed Angle

Swinging Arm

Centrifuge Rotors axis of rotation Swinging-bucket At rest Spinning g g Fixed-angle

Geometry of Rotors rmax rav rmin axis of rotation a rmin rav rmin rav rmax rmax b c Sedimentation path length

Stokes Equation 2 v d ( p l ) 18 v velocity of sedimentation p density of particle g centrifugal force g d diameter of particle l density of liquid viscosity of liquid

Use of a NOMOGRAM

Use in Lab Microfuge Low Speed High Speed 15,000 rpm 10,000 rpm 30,000 rpm 21k x g 8k x g 100k x g Separate precipitated DNA from supernatant, drive all liquid to the bottom of a tube Separate solid pellet from liquid supernatant; pellet whole cells from liquid medium Separate major components of cells from each other; separate mitochondria, nuclei, each

Types of Centrifugation Differential Centrifugation: Rate-Zonal Centrifugation Isopycnic Centrifugation: Density Equilibrium Centrifugation

Differential Centrifugation This is the most common method of fractionating cells Fractionation is the separation of the different organelles within the cell

Differential Centrifugation Density of liquid is uniform Density of liquid Density of particles Viscosity of the liquid is low Consequence: Rate of particle sedimentation depends mainly on its size and the applied G-force.

Size of Major Cell Organelles Nucleus 4-12 m Plasma membrane sheets 3-20 m Golgi tubules 1-2 m Mitochondria 0.4-2.5 m Lysosomes/peroxisomes 0.4-0.8 m Microsomal vesicles 0.05-0.3 m

Differential Centrifugation of a Tissue Homogenate 1000g/10 min etc. 3000g/10 min Decant supernatant

1,000 g (1,000 times the force of gravity) 10 min Supernatant poured into next tube 20,000 g 20 min 80,000 g 60 min Pellet rich in nuclei and cellular debris 150,000 g 3 hr Pellet rich in mitochondria (and chloro-plasts if cells are from a plant) Pellet rich in “microsomes” (pieces of plasma membranes and cells’ internal membranes) Pellet rich in ribosomes

Cell Fractionation by Differential Centrifugation

Differential Centrifugation Poor resolution and recovery because of: – Particle size heterogeneity – Particles starting out at rmin have furthest to travel but initially experience lowest RCF – Smaller particles close to rmax have only a short distance to travel and experience the highest RCF

Differential Centrifugation Swinging-bucket rotor: Long sedimentation path length gmax gmin Fixed-angle rotor: Shorter sedimentation path length, gmax gmin

Density Gradient Centrifugation Density Barrier Discontinuous Continuous

How Does a Gradient Separate Different Particles? Least dense Most dense

Stokes Equation 2 v d ( p l ) 18 When p l : v is ve When p l : v is 0 When p l : v is –ve g

1 Buoyant density banding 2 3 4 5 Equilibrium density banding Isopycnic banding

3 Formats for Separation of Particles According to Density 1 2 3 When density of particle density of liquid V is -ve

Resolution of Density Gradients Density Barrier I II Discontinuous Continuous

Organelle Separation by Isopycnic Centrifugation

Isopycnic Centrifugation The density gradient is formed during the centrifugation. The sample is dissolved in solution of dense salt (e.g., CsCl, Cs2(SO4)) The macromolecules of the biological sample seek an area in the tube where the density is equal to their respective densities

Densities of Biological Material Material Density (g/cm3) Microbial cells 1.05 - 1.15 Mammalian cells 1.04 - 1.10 Organelles 1.10 - 1.60 Proteins 1.30 DNA 1.70 RNA 2.00

Separation of Particles According to Size p l : v is ve for all particles throughout the gradient

SAFETY during Centrifugation A rotor spinning at high speed is DANGEROUS Always balance the tubes, to avoid damaging the centrifuge or yourself. Never open a centrifuge that is spinning Centrifuges generate aerosols

SAFETY

Balance your Tubes, Always!!

Load Tubes Evenly

Organelle Separation Procedure Cut tissue in an ice-cold isotonic buffer. It is cold to stop enzyme reactions, isotonic to stop osmosis and a buffer to stop pH changes. Grind tissue in a blender to break open cells. Filter to remove insoluble tissue

Quantitation of Concentration UV/vis Absorption Limitation of Beer-Lambert Equation Correct Choice of Wavelength Spec 20

Beer’s Law source slit cuvette detector A -logT log(P0/P) ecL T Psolution/Psolvent P/P0 Works for monochromatic light Compound x has a unique e at different wavelengths

Color of the sample Remember: – Solution absorbs red appears bluegreen – Solution absorbs blue-green appears red

Types of Electronic Transition Three types – Involving p, s, and n electrons – Involving d and f orbital electrons – Charge transfer electrons

Beer’s Law Analysis Choice of wavelength – Typically choose wavelength of maximum absorbance – May deviate from this to avoid an interference

Different Shapes and Sizes of Cells

Cuvette The cuvettes for use in the Spec 20 resemble small test tubes. – Each cuvette is marked so that it can be positioned properly in the sample holder. – The mark is at the top of the cuvette and must be positioned toward the front of the spectrophotometer when taking measurements. – Handle these tubes with extreme care to keep both the inside and outside surfaces clean and free of scratches. Cleaning the Cuvette: – NEVER use a brush to clean the inside of the cuvette. – Rinse the tube with distilled water a few times – Add about 1 mL of the solution to be measured. Tilt and turn the cuvette so that the solution has contact with all the surfaces. Discard this solution and repeat this rinse once more. – Fill the cuvette about 3/4 full of the solution you wish to test. – Wipe the outside of the cuvette with a lint-free, soft tissue (a Chemwipe) to remove any moisture or fingerprints from the outside surface.

Spectronic 20 W filament light source Grating dispersion 340 - 640 nm at 20 2.5 nm

Overview of Spectronic 20 Spectronic 20 is a spectrophotometer. A spectrophotometer measures the intensity of a light beam before and after it passes through a sample and compares these two intensities. The Spec 20 reports two types of measurements: percent transmittance (%T) and absorbance (A). – Percent transmittance is the ratio of the intensity of the light passing through the sample to the intensity of the light shining on the sample multiplied by 100%. – Absorbance is the log of the transmittance. – The Spec 20 can measure absorbance and transmittance over a range of wavelengths. You must select a wavelength and calibrate the instrument at that wavelength before making any measurements.

Generic Operating Instructions Connect the Spectronic 20 to an electrical outlet. – Turn on the instrument with the zero adjust/on-off control and allow about five minutes warm-up time. – Turn the wavelength control knob to select the desired wavelength. Calibration 0 %T – With no sample tube in place and the cover closed, turn the zero adjust knob to bring the meter to 0% transmittance. Calibration 100 %T – Fill the spectrophotometer tube to about 2/3 with distilled water. Make sure that the outside of the tube is clean and dry (do not handle the tube on the sides so as to avoid fingerprints) and insert it in the cuvette holder, pushing it all the way down and aligning it so that the reference line on the cuvette holder matches a line (or other mark) on the tube. Close the cover of the cuvette holder. – Adjust the light control knob on the spectrophotometer so that the meter reads 100% transmittance. calibration should be repeated before each absorbance measurement.

Measuring the Sample Fill (to 2/3) a tube with the solution to be determined. – Wipe the outside of the tube to remove water and fingerprints and insert into the cuvette holder, aligning as above. Close the cover and read the % transmittance (or absorbance) from the meter scale. The same sample tube should be used for all absorbance measurements. – Care must be taken to align the tube in exactly the same way each time. – A separate tube may be used for the distilled water blank, but again always align it in the same way. Because the sample may be heated in the light beam or photochemical decomposition may occur, – the absorbance reading should be made quickly after the tube is inserted. It is good practice to read and record the % transmittance meter reading and to convert to absorbance later. – The transmittance scale is linear and thus less subject to reading error than the logarithmic scale.

Calibrating the Spectronic 20 Plug in and turn on the Spec 20. It must warm up for 30 minutes before use. A schematic of an older instrument is shown at the left. Set the instrument to the proper wavelength by turning the knob located on the right hand surface of the spectrophotometer. The wavelength setting can be seen through the window next to the knob. Obtain a properly cleaned cuvette and fill it about 3/4 full of the reference solution (usually water). With no cuvette in the sample holder, close the cover and rotate the zero light control knob (left front knob) to display a reading of 0.0% transmittance. Provided that the instrument is not turned off and this knob is not moved, no other adjustments to this control are needed. Place the reference solution cuvette in the sample holder, close the cover, and rotate the light control knob (front right knob) to display a reading of 100.0% transmittance. This procedure must be repeated every time measurements are taken at a new wavelength or if several measurements are made at the same wavelength.

Requirement for Report Tabulate all the absorbance of each tube measured. Tabulate all the concentration of chlorophyll in each tube. Graph the relationship between absorbance and tube number Graph the concentration vs. tube number. Attention: The unit of concentration.







