C1- Chemicals 1) Methanol (1) 제 법 : ① ~1913 BASF: During NH3
21 Slides141.50 KB
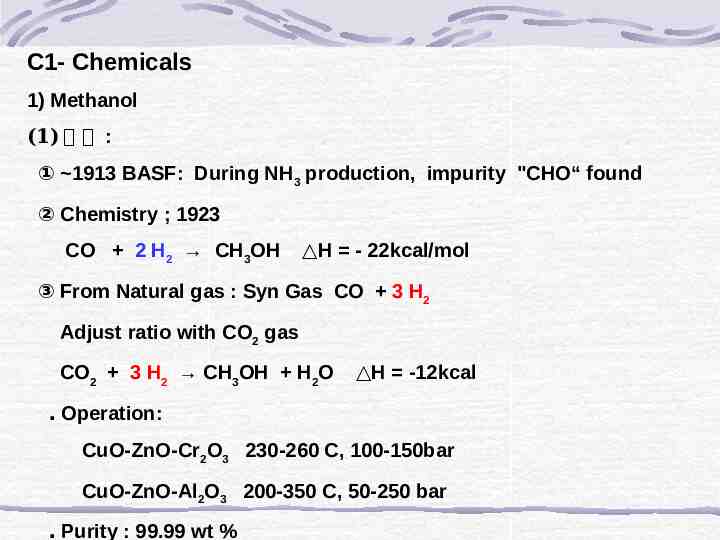
C1- Chemicals 1) Methanol (1) 제 법 : ① 1913 BASF: During NH3 production, impurity "CHO“ found ② Chemistry ; 1923 CO 2 H2 CH3OH H - 22kcal/mol ③ From Natural gas : Syn Gas CO 3 H2 Adjust ratio with CO2 gas CO2 3 H2 CH3OH H2O H -12kcal ․ Operation: CuO-ZnO-Cr2O3 230-260 C, 100-150bar CuO-ZnO-Al2O3 200-350 C, 50-250 bar ․ Purity : 99.99 wt %

(2) 용도 : Chemicals (70%) Fuel (30%) ① Formaldehyde ② HOAc, AC2O ③ MMA ④ Dimethyl Terephthalate (DMT) ⑤ Solvent ⑥ 기타 (MTBE): Gasolin additive for high O.N (3) Future Application : Mobile MTG (methanol to gasoline)process ① Feed for Synthesis CH3OH H2O CH3OCH3 CH3OH ZSM-5 CH3(CH2 )nCH3 (Aromatics H2O) ※ Geometry of zeolite prevents formation of aromatics
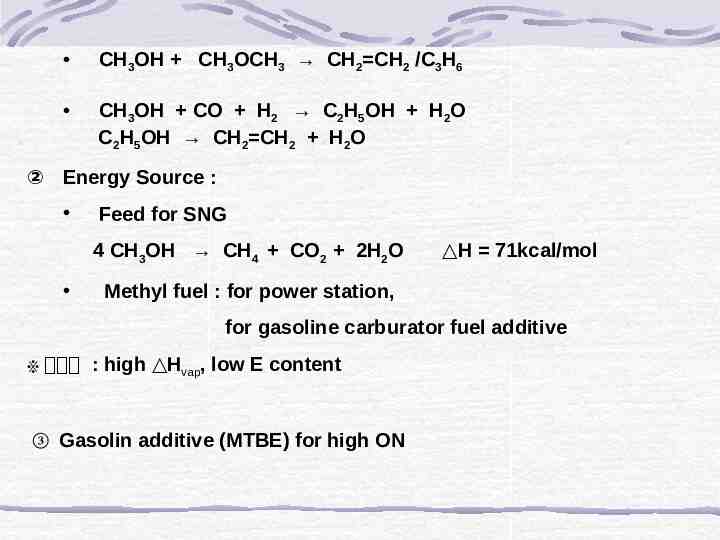
CH3OH CH3OCH3 CH2 CH2 /C3H6 CH3OH CO H2 C2H5OH H2O C2H5OH CH2 CH2 H2O ② Energy Source : Feed for SNG 4 CH3OH CH4 CO2 2H2O H 71kcal/mol Methyl fuel : for power station, for gasoline carburator fuel additive ※ 문제점 : high Hvap, low E content ③ Gasolin additive (MTBE) for high ON

2) Formaldehyde (1) Physical Property ① aq solution (35-55%) HCHO․H2O : HO-CH2-OH (99%) H-(OCH2)n-OH : n 〈 10 ② Trioxan 3 HCHO trioxane ③ Paraformaldehyde : n HCHO H-(OCH2)n-OH : n 10

(2) Source : ① CH3OH ; 92% ② C1-C4 Alkane ; 8% ③ CH3OCH3 (3) 합성법 ※ Two route : - Oxidative dehydration of MeOH / [Ag or Cu] - Oxidation of MeOH / [Fe-MoO3]

① CH3OH HCHO H2 H2 ½ O 2 H 2O H 20kcal H -58kcal - Net Reaction CH3OH 0.5O2 HCOH H2O H -38kcal ( 유진화학 ) ․ By product : CO, CO2 : no formic acid ! ․ Reaction condition - Temp ; 600 720 5 - O2 addition ; little less than stoichiometry - H2O added to complete reaction (advantage) - increase CH3OH conversion - Ag sintering 제어 (2-4 mouth life) - lowers C deposition on Ag

② CH3OH air HCOH Others (CO, CO2, formic acid) ․ Reaction condition - Temp ; 350 450 - Fe2O3/MoO3 95-99% conversion, 91-94% selectivity ③ CH4 or C3/C4 air HCOH, CH3COH, . (4) 용도 ; ① Formaldehyde condensation product : thermosetting product ② Trioxan (from water free HCOH) ③ Aldol rxn prod: eg) Pentaerythritol 등 ④ Glycolic acid (Dupont) HCHO CO H2O HOCH2COOH HOCH2COOCH3 EG (NG)

3) Formic acid (1) 합성법 : ① CO H2O HCOOH CO ROH HCOOR ․ 35% of world production ․ Base catalyzed reaction of CO (under press) with H2O to acid with ROH to ester (ROH MeOH ; 70 , 20-200 bar) ․ Free HCOOH obtained via - Direct ester hydrolysis - Ammonolysis & hydrolysis eg) CH3OH CO HCOOCH3 NH3 HCONH2 CH3OH HCONH2 H HCOOH (NH4)2SO4 ※prevents retro-esteritication

② As by-prod of "HC" Oxidation - Light naphtha or butane for HOAc production - 60% of world production (2) 용도 ① Fermentation : pH adjusting agent Na, Al Salt : 가죽, textile industry ② 2 HCOONa NaOH (COONa)2 H2 ③ Introduction of formyl group in Org Synthesis eq) Vitamine B1 ④ Amides (as solvents) - HCO2CH3 NH2CH3 (or NH(CH3)2) HCONHCH3 (or DMF) cf: NH(CH3)2 CO NaOCH3 HCON(CH3)2 (or DMF)
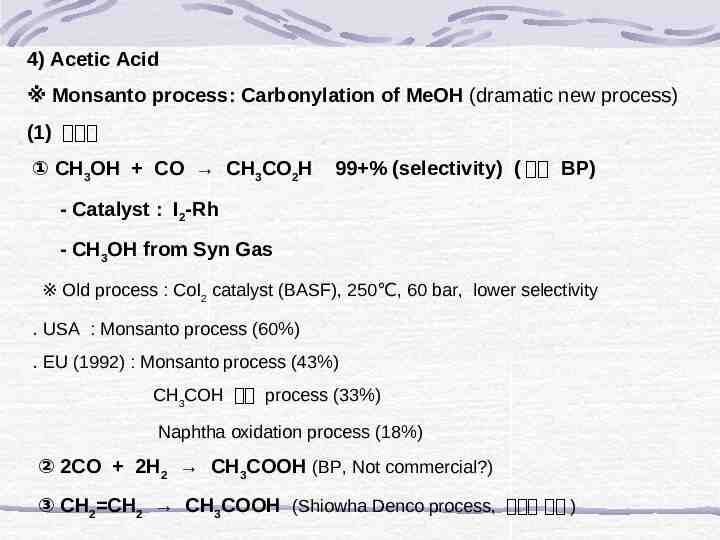
4) Acetic Acid ※ Monsanto process: Carbonylation of MeOH (dramatic new process) (1) 합성법 ① CH3OH CO CH3CO2H 99 % (selectivity) ( 삼성 BP) - Catalyst : I2-Rh - CH3OH from Syn Gas ※ Old process : CoI2 catalyst (BASF), 250 , 60 bar, lower selectivity ․ USA : Monsanto process (60%) ․ EU (1992) : Monsanto process (43%) CH3COH 산화 process (33%) Naphtha oxidation process (18%) ② 2CO 2H2 CH3COOH (BP, Not commercial?) ③ CH2 CH2 CH3COOH (Shiowha Denco process, 공기반 과제 )
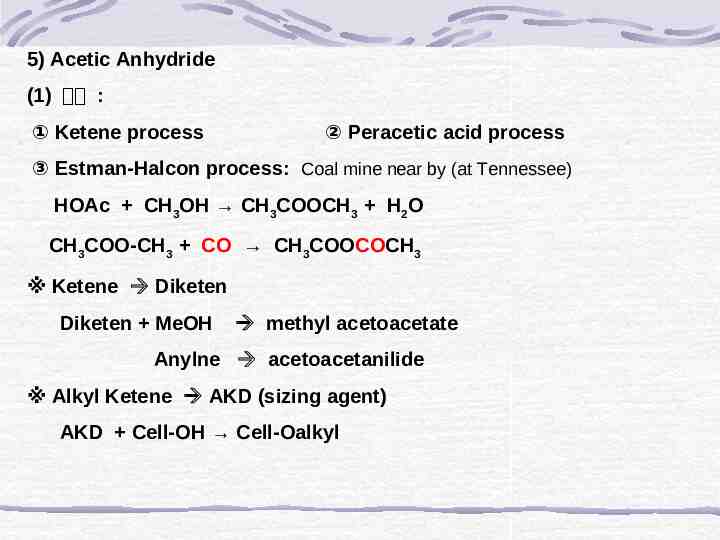
5) Acetic Anhydride (1) 제법 : ① Ketene process ② Peracetic acid process ③ Estman-Halcon process: Coal mine near by (at Tennessee) HOAc CH3OH CH3COOCH3 H2O CH3COO-CH3 CO CH3COOCOCH3 ※ Ketene Diketen Diketen MeOH methyl acetoacetate Anylne acetoacetanilide ※ Alkyl Ketene AKD (sizing agent) AKD Cell-OH Cell-Oalkyl

(2) 용도 : Acetates ※ First & only process /replaced petroleum based process Coal O2 H2O CO H2 CO H2 CH3OH HOAc CH3OH CH3COOCH3 H2O CH3COOCH3 CO CH3COOCOCH3 (Ac2O) Ac2O Cell-OH Cell-OAc HOAc cf: High cost of Gasifies (3 Expensive) cheap Coal

6) HCN (1) 합성법 : ① Direct Synthesis: ․ Dehydration of formamide HCONH2 HCN H2O ․ Oxidative or dehydrogenative rxn of NH3 w/ “HC” (esp CH4) CH4 NH3 1.5O2 HCN 3H2O CH4 NH3 [Catal, w/out O2] HCN 3H2 ․ CO NH3 HCN H2O ② As by product : "Sohio Process" - Acrylonitrile by-product from propylene - 100,000t/y vs 183,000t/y (USA) by "direct synthesis"
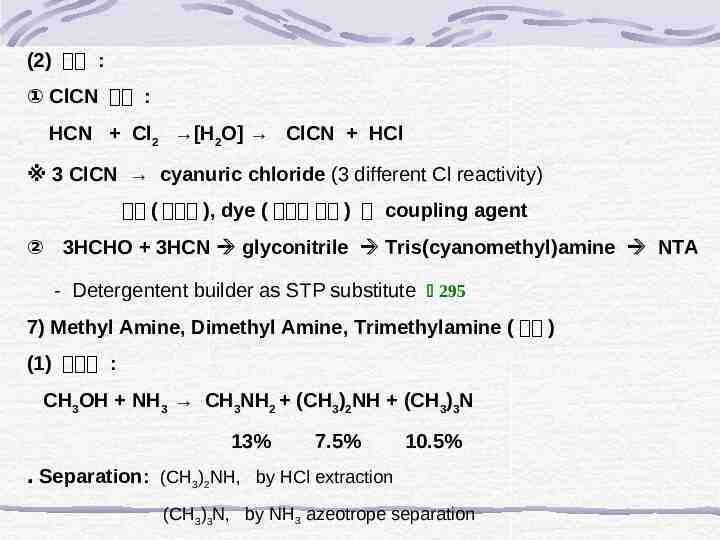
(2) 용도 : ① ClCN 생산 : HCN Cl2 [H2O] ClCN HCl ※ 3 ClCN cyanuric chloride (3 different Cl reactivity) 농약 ( 제초제 ), dye ( 반응성 염료 ) 의 coupling agent ② 3HCHO 3HCN glyconitrile Tris(cyanomethyl)amine NTA - Detergentent builder as STP substitute 295 7) Methyl Amine, Dimethyl Amine, Trimethylamine ( 소량 ) (1) 합성법 : CH3OH NH3 CH3NH2 (CH3)2NH (CH3)3N 13% 7.5% 10.5% ․ Separation: (CH3)2NH, by HCl extraction (CH3)3N, by NH3 azeotrope separation

(2) 용도 : ① 용매 (CH3)2NH CO (CH3)2NCOH (DMF) ( 삼성 종합화학 , 1999) (CH3)2NH HOAc (CH3)2NCOCH3 (DMA) H2O ② CH3NH2 COCl2 CH3N C O (MIC) 2HCl MIC ROH carbamate 계 농약 (Bhophal: disaster in Chem Ind. History, 327) ③ (CH3)3N EO Choline chloride (choline, animal feed supp) 8) Dimethyl carbonate ( 소량 ) (1) 합성법 : 2 CH3OH 1/2O2 CO CH3O-CO-OCH3 (2) 용도 : phosgene, DMS (for isocyanate, alkylation 대용품 ) - Polycarbonate w/out phosgen - Alkylation
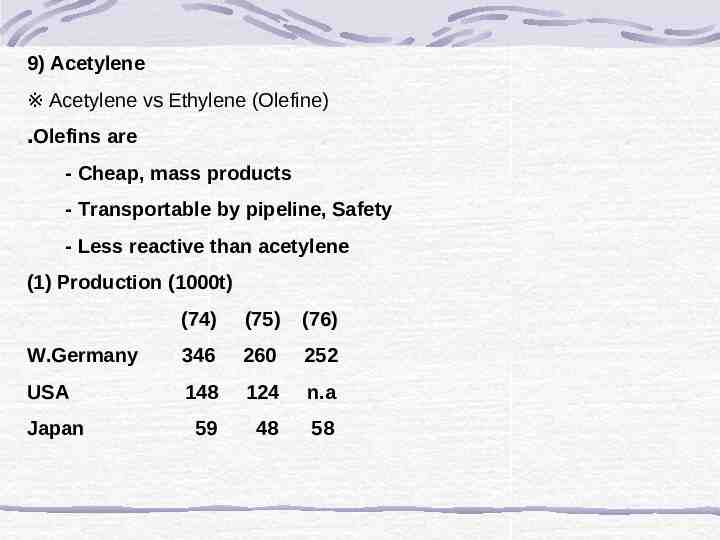
9) Acetylene ※ Acetylene vs Ethylene (Olefine) ․Olefins are - Cheap, mass products - Transportable by pipeline, Safety - Less reactive than acetylene (1) Production (1000t) (74) (75) (76) W.Germany 346 260 252 USA 148 124 n.a 59 48 58 Japan

(2) Olefine 으로 대체된 품목 : ① CH2 CH2 : CH3COH (CH CH H2O); VCM (CH CH HCl) CH CHOAc (CH CH HOAc), CCl2 CHCl, CCl2 CCl2 ② Propylene : CH2 CHCN (CH CH HCN) CH2 CHCOOH (CH CH ROH CO) acrylate ③ Butadiene : CH2 C(Cl)-CH CH2 (CH C-CH CH2) (3) 제조법 : CaC2 Based Process CaO(lime) C(coke) CaC2 CO CaC2 2H2O CH CH Ca(OH)2 ( origin of Union Carbide)

※Main cost factors - Batch process, Coal (solid) - Labor intensive(solid raw material) - 환경문제 : 1t 의 CH CH 생산 2.8t Ca(OH)2(slurry in 28ton H2O) - Life time of furnace is short - High E. cost : 10kwh/kg CH CH (4) 제조법 :Thermal process - Feed : CH4 Crude oil - Very high Temp pyrolysis : 〉 1400 - Very Short Reation Time : 0.01 0.001 sec - Rapid quenching

① Electric arc process - Allothermal process w/ direct heat transfer (electrical heating) - Feed : HC(b.p up to 200 ) Cracked in 1m long electric arc ② Partial Oxidation Process (Direct Autothermal Cracking) ․ O2 (pre heated) CH4(pre heated) (incomplete Ox, pyrolysis, 10-3sec ) Quenching

(5) 용도 : ① CH CH HCHO HOCH2-C C-CH2OH 1,4 Butandiol (Reppe) 1,4 Butandiol THF Poly(tetramethylene ether glycol) PU (Spandex)
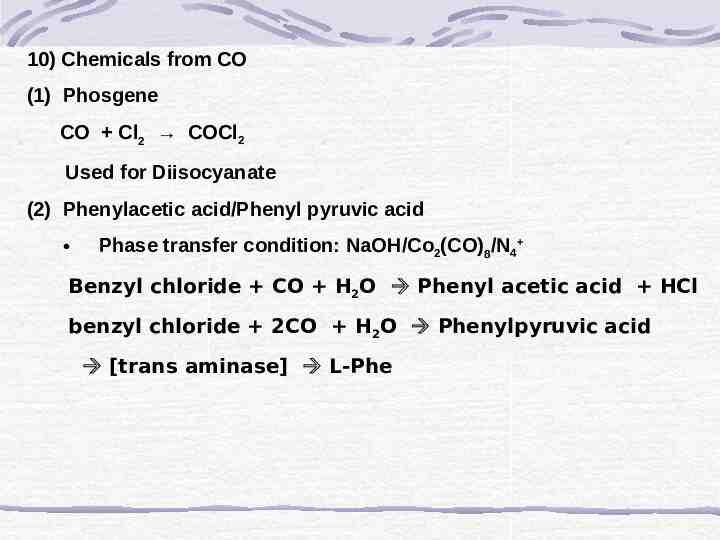
10) Chemicals from CO (1) Phosgene CO Cl2 COCl2 Used for Diisocyanate (2) Phenylacetic acid/Phenyl pyruvic acid Phase transfer condition: NaOH/Co2(CO)8/N4 Benzyl chloride CO H2O Phenyl acetic acid HCl benzyl chloride 2CO H2O Phenylpyruvic acid [trans aminase] L-Phe






