The Future of HHS Quality and Payment Policies: Beyond Meaningful Use
96 Slides5.62 MB

The Future of HHS Quality and Payment Policies: Beyond Meaningful Use Transforming the Provision of Healthcare and the use of Health IT October 6, 2015 Julia Skapik, MD, MPH Medical Officer Office of the National Coordinator for Health IT

Objectives Discuss the movement of Meaningful Use from Stage 2 to Stage 3 Discuss recent Congressional legislation and other proposed changes to quality measurement and payment The role of interoperability in quality and payment moving forward Learn about the current standards strategy for health IT in the US government moving towards this new vision from HHS 2

The Future of HHS Quality and Payment Policies: The Federal Government and its Current and Future Role in Health IT Julia Skapik, MD, MPH Medical Officer Office of the National Coordinator for Health IT
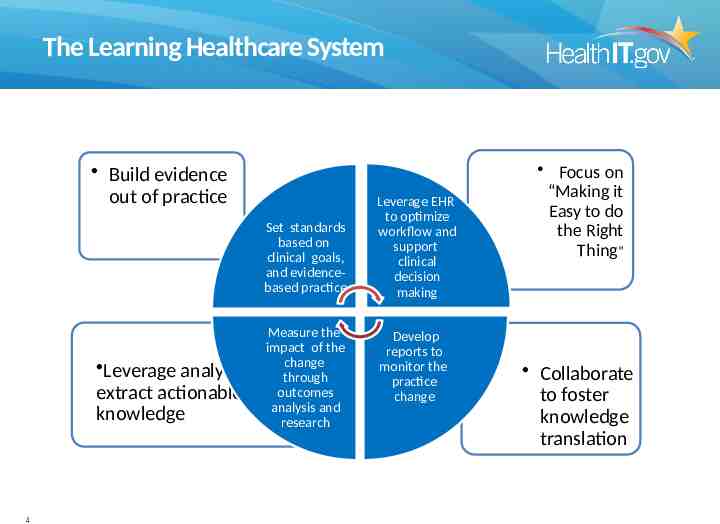
The Learning Healthcare System Build evidence out of practice Set standards based on clinical goals, and evidencebased practice Leverage analytics extract actionable knowledge 4 Measure the impact of the change to through outcomes analysis and research Leverage EHR to optimize workflow and support clinical decision making Develop reports to monitor the practice change Focus on “Making it Easy to do the Right Thing” Collaborate to foster knowledge translation

Objectives Discuss the movement of Meaningful Use from Stage 2 to Stage 3 Discuss recent Congressional legislation and other proposed changes to quality measurement and payment The role of interoperability in quality and payment moving forward Learn about the current standards strategy for health IT in the US government moving towards this new vision from HHS 5

ONC Certification 2015 Edition Proposed Rule Criteria 6
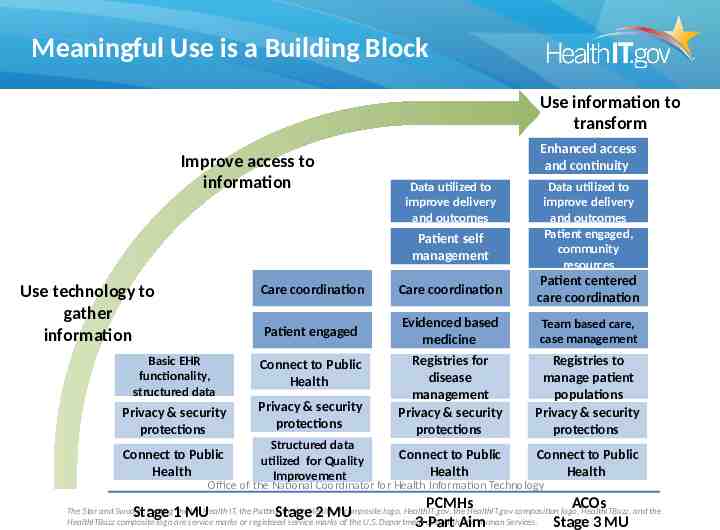
Meaningful Use is a Building Block Use information to transform Improve access to information Enhanced access and continuity Data utilized to improve delivery and outcomes Patient self management Use technology to gather information Data utilized to improve delivery and outcomes Patient engaged, community resources Care coordination Care coordination Patient centered care coordination Patient engaged Evidenced based medicine Team based care, case management Registries to manage patient populations Privacy & security protections Connect to Public Health Basic EHR functionality, structured data Connect to Public Health Privacy & security protections Privacy & security protections Registries for disease management Privacy & security protections Connect to Public Health Structured data utilized for Quality Improvement Connect to Public Health Stage 2 MU PCMHs 3-Part Aim Office of the National Coordinator for Health Information Technology Stage 1 MU ACOs Stage 3 MU The Star and Swoosh, Putting the I in Health IT, the Putting the I in Health IT composite logo, HealthIT.gov, the HealthIT.gov composition logo, HealthITBuzz, and the HealthITBuzz composite logo are service marks or registered service marks of the U.S. Department of Health and Human Services.

Proposed EHR Incentive Program Stage 3 Meaningful Use and Certification Modular certification Increased testing robustness Updated standards and data capture rules Incremental increases in requirements for electronic-based care activities Moving towards outcome measurement Increasing specificity of exchange requirements 8

Supporting the Broader Care Continuum: How Would It Work? The Past (2011 and 2014 Editions) The Proposed Future (2015 and Future Editions) ONC included policy that supported ONC does not include policy to support the the EHR Incentive Programs in its EHR Incentive Programs in its Editions previous Editions Each program sets its own requirements (e.g., CMS defines the CEHRT definition in Defined the Certified EHR Technology (CEHRT) definition its rule) on behalf of CMS ONC’s Health IT Certification Program is “agnostic” to settings and programs, but Required “meaningful use measurement” criteria can support many different use cases and needs Specified the minimum number of clinical quality measures This allows ONC’s Health IT Certification developers must certify to in Program to support multiple program order to participate in the EHR and setting needs, for example: Incentive Programs EHR Incentive Programs Specified criteria as Long-term and post-acute care “ambulatory” or “inpatient” Chronic care management Behavioral health Other public and private programs 9

Other Programs Using the ONC Health IT Certification Program Proposed: A more accessible ONC Health IT Certification Program supportive of: Diverse health IT systems, including but not limited to EHR technology (“Health IT Module” instead of “EHR Module”) Remember that there is no “Complete EHR” certification to the 2015 Edition or future editions Health IT across the care continuum, including long-term and post acute care settings New possible settings using ONC Certification 2015 Edition and Beyond: Physician Self-Referral Law exception and Anti-kickback Statute safe harbor for certain EHR donations CMS chronic care management services Department of Defense Healthcare Management System Modernization Program The Joint Commission for participation as ORYX vendor – eCQMs for hospitals 10
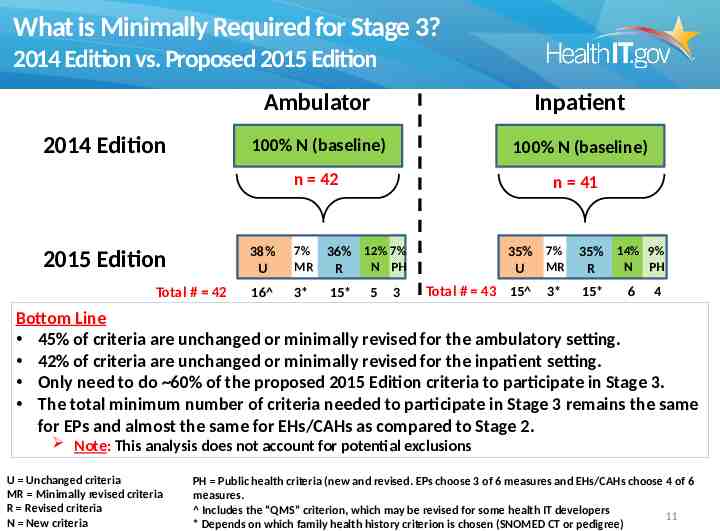
What is Minimally Required for Stage 3? 2014 Edition vs. Proposed 2015 Edition Ambulator y 2014 Edition 2015 Edition Total # 42 Inpatient 100% N (baseline) 100% N (baseline) n 42 n 41 38% U 7% MR 36% 12% 7% N PH R 16 3* 15* 5 3 35% 7% MR U Total # 43 15 3* 35% 14% 9% N PH R 15* 6 4 Bottom Line 45% of criteria are unchanged or minimally revised for the ambulatory setting. 42% of criteria are unchanged or minimally revised for the inpatient setting. Only need to do 60% of the proposed 2015 Edition criteria to participate in Stage 3. The total minimum number of criteria needed to participate in Stage 3 remains the same for EPs and almost the same for EHs/CAHs as compared to Stage 2. Note: This analysis does not account for potential exclusions U Unchanged criteria MR Minimally revised criteria R Revised criteria N New criteria PH Public health criteria (new and revised. EPs choose 3 of 6 measures and EHs/CAHs choose 4 of 6 measures. Includes the “QMS” criterion, which may be revised for some health IT developers 11 * Depends on which family health history criterion is chosen (SNOMED CT or pedigree)

2015 Edition Specific Health IT Goals Improve Interoperability Ensure Privacy and Security Capabilities Reduce Health Disparities Use the ONC Health IT Certification Program to Support the Care Continuum Facilitate Data Access and Exchange Improve Patient Safety Improve the Reliability and Transparency of Certified Health IT Support Stage 3 of the EHR Incentive Programs 12
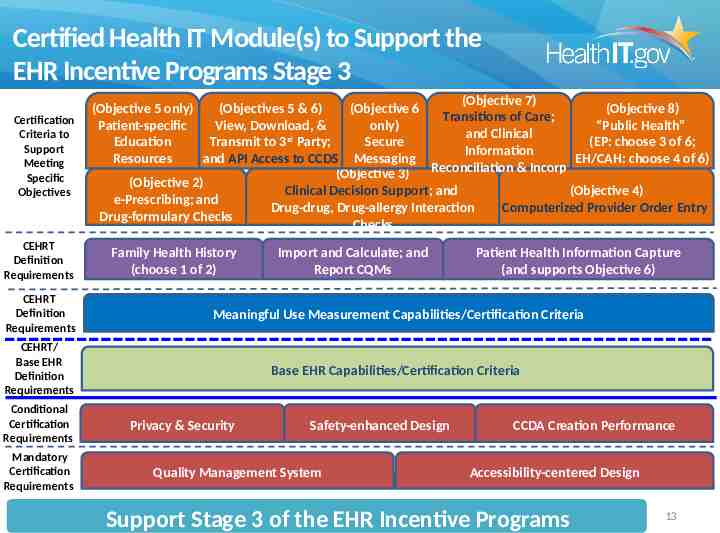
Certified Health IT Module(s) to Support the EHR Incentive Programs Stage 3 Certification Criteria to Support Meeting Specific Objectives CEHRT Definition Requirements (Objective 7) (Objective 5 only) (Objectives 5 & 6) (Objective 6 (Objective 8) Transitions of Care; Patient-specific View, Download, & only) “Public Health” and Clinical rd Education Transmit to 3 Party; Secure (EP: choose 3 of 6; Information Resources and API Access to CCDS Messaging EH/CAH: choose 4 of 6) Reconciliation & Incorp (Objective 3) (Objective 2) Clinical Decision Support; and (Objective 4) e-Prescribing; and Drug-drug, Drug-allergy Interaction Computerized Provider Order Entry Drug-formulary Checks Checks Family Health History (choose 1 of 2) Import and Calculate; and Report CQMs Patient Health Information Capture (and supports Objective 6) CEHRT Definition Requirements Meaningful Use Measurement Capabilities/Certification Criteria CEHRT/ Base EHR Definition Requirements Base EHR Capabilities/Certification Criteria Conditional Certification Requirements Mandatory Certification Requirements Privacy & Security Safety-enhanced Design Quality Management System CCDA Creation Performance Accessibility-centered Design Support Stage 3 of the EHR Incentive Programs 13

2015 Base EHR Definition * red new to the Base EHR Definition ** privacy and security removed – now conditional certification requirements Base EHR Capabilities Certification Criteria Includes patient demographic and clinical health information, such as medical history and problem lists Demographics § 170.315(a)(5) Problem List § 170.315(a)(7) Medication List § 170.315(a)(8) Medication Allergy List § 170.315(a)(9) Smoking Status § 170.315(a)(12) Implantable Device List § 170.315(a)(20) Capacity to provide clinical Clinical Decision Support § 170.315(a)(10) decision support Capacity to support Computerized Provider Order Entry (medications, laboratory, or diagnostic imaging) § 170.315(a)(1), (2) or (3) physician order entry Capacity to capture and query information Clinical Quality Measures (CQMs) – record and export § 170.315(c)(1) relevant to health care quality Capacity to exchange Transitions of Care § 170.315(b)(1) electronic health Data Portability § 170.315(b)(6) information with, and Application Access to Common Clinical Data Set § 170.315(g)(7) integrate such information Direct Project § 170.315(h)(1) or Direct Project, Edge Protocol, and from other sources XDR/XDM § 170.315(h)(2) 14
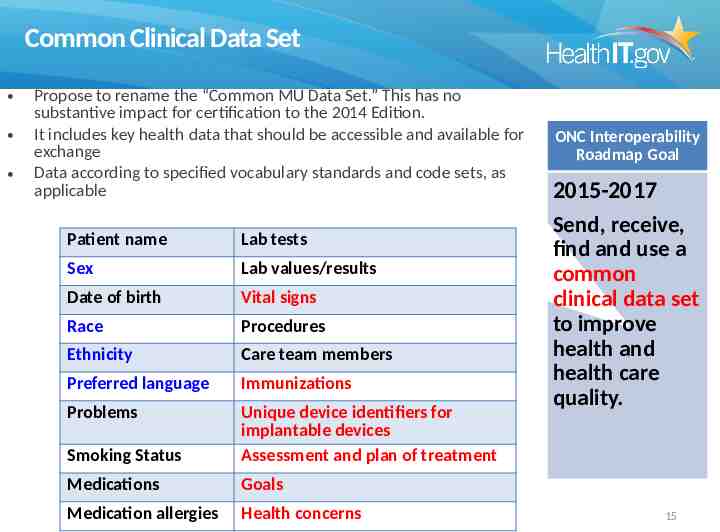
Common Clinical Data Set Propose to rename the “Common MU Data Set.” This has no substantive impact for certification to the 2014 Edition. It includes key health data that should be accessible and available for exchange Data according to specified vocabulary standards and code sets, as applicable Patient name Lab tests Sex Lab values/results Date of birth Vital signs Race Procedures Ethnicity Care team members Preferred language Immunizations Problems Smoking Status Unique device identifiers for implantable devices Assessment and plan of treatment Medications Goals Medication allergies Health concerns ONC Interoperability Roadmap Goal 2015-2017 Send, receive, find and use a common clinical data set to improve health and health care quality. 15
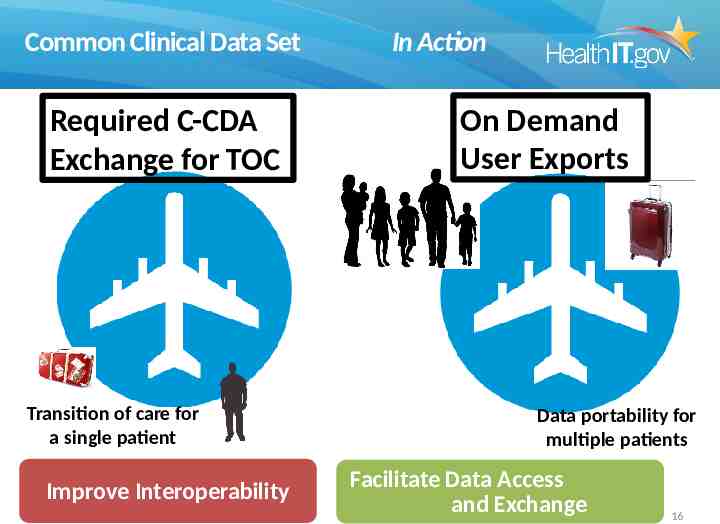
Common Clinical Data Set Required C-CDA Exchange for TOC Transition of care for a single patient Improve Interoperability In Action On Demand User Exports Data portability for multiple patients Facilitate Data Access and Exchange 16

Application Access to CCDS Requirements 1) 2) 3) Security -- developer demonstrates a trusted connection can be established between source system's API and other software Patient selection – means for an application to query for a patient’s record Data -- scope is limited to the data in CCDS per patient and a "get"/read-oriented request. Must support: Data-category request (response format in XML/JSON) All data request (response format in accordance with the Consolidated CDA) 4) Documentation Must include accompanying documentation on technical implementation requirements Must include terms of use, including developer agreements Request for Comment 4) 5) 6) How to foster an open ecosystem around APIs Whether additional API capabilities should be required for certification Should the C-CDA 2.0 creation capability be limited to the CCD document template Improve Interoperability Facilitate Data Access and Exchange 17

Addressing Health Disparities Proposed Certification Criteria Capabilities What the Capabilities Provide More granular recording and exchange of patient race and ethnicity Allows providers to better understand health disparities based on race and ethnicity, and improve patient care and health equity. Recording social, psychological, and behavioral data (e.g., education level, stress, depression, alcohol use, sexual orientation and gender identity) Allows providers and other stakeholders to better understand how this data can affect health, reduce disparities, and improve patient care and health equity Exchange of sensitive health information (data segmentation for privacy) Allows for the exchange of sensitive health information (e.g., behavioral health, substance abuse, and genetic information), in accordance with federal and state privacy laws, for more coordinated and efficient care across the continuum. Accessibility of health IT More transparency on the accessibility standards used in developing health IT Compatibility of certified health IT with accessibility technology (e.g., JAWS text-to-speech application) Web content accessibility for viewing capability of VDT Reduce Health Disparities 18

Delta’s between the 2014 Edition & proposed 2015 Edition 2014 Edition Quality management system 2015 Edition Difference Removed option to meet the criterion by attesting to using “no” QMS. All privacy and security criteria Wording clarifications, but no substantive changes Safety-enhanced design Expanded list of applicable certification criteria 19
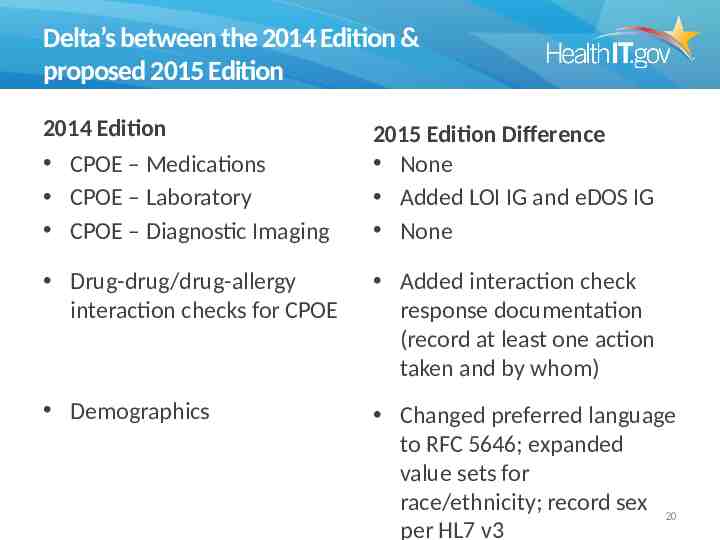
Delta’s between the 2014 Edition & proposed 2015 Edition 2014 Edition CPOE – Medications CPOE – Laboratory CPOE – Diagnostic Imaging 2015 Edition Difference None Added LOI IG and eDOS IG None Drug-drug/drug-allergy interaction checks for CPOE Added interaction check response documentation (record at least one action taken and by whom) Demographics Changed preferred language to RFC 5646; expanded value sets for race/ethnicity; record sex 20 per HL7 v3

Delta’s between the 2014 Edition & proposed 2015 Edition 2014 Edition 2015 Edition Difference Vital Signs (not associated with Stage 3) Added additional vital signs; record vital signs according to LOINC and UCUM; record metadata; offer optional certification, including for pediatrics Problem List Set baseline as the most recent version of SNOMED CT 21

Delta’s between the 2014 Edition & proposed 2015 Edition 2014 Edition Clinical Decision Support Drug-formulary and preferred drug list checks Smoking status 2015 Edition Difference Updated Infobutton standards; Added interaction check response documentation (record at least one action taken and by whom) Adopt NCPDP Formulary and Benefit Standard v3.0 (if drugformulary); auto-check whether DF or PDL exists for patient and medication; indicate last update of DF of PDL Record, change, and access smoking status in any SNOMED CT code (but must be able to exchange using 8 specified SNOMED CT codes) 22

Delta’s between the 2014 Edition & proposed 2015 Edition 2014 Edition 2015 Edition Difference Family health history Updated SNOMED CT Family health history – pedigree HL7 Pedigree standard and HL7 Pedigree IG Only require Infobutton; updated Patient-specific education Infobutton standards; do not have resources to use Infobutton for lab values/results; request resources based on patient’s preferred language Transitions of care Refer to description of proposal in previous slides Data portability Refer to description of proposal in previous slides 23

Delta’s between the 2014 Edition & proposed 2015 Edition 2014 Edition 2015 Edition Difference Clinical information Reconcile problem, med, med allergy data from valid C-CDAs (Releases 1.1 and reconciliation and incorporation E-prescribing (e-Rx) View, download, and transmit to 3rd party 2.0); generate conformant C-CDA based on reconciled info Expand e-Rx transactions; ability to codify e-Rx instructions in structured Sig format; e-prescribe all meds in metric unit standard Updated C-CDA R2; updated Common Clinical Data Set; make diagnostic imaging reports available for patient certification; require API access capabilities (similar to App Access to CCDS); add “addressee” to activity history log; provide lab reports in compliance with CLIA 24

Delta’s between the 2014 Edition & proposed 2015 Edition 2014 Edition CQMs – record and export CQMs – import and calculate 2015 Edition Difference For all CQM criteria – comment solicitation on the versions of standards to adopt User “on demand” ability to export data User “on demand” ability to import data; “closed” systems must demonstrate import capability; intent to test import of larger # of test records 25
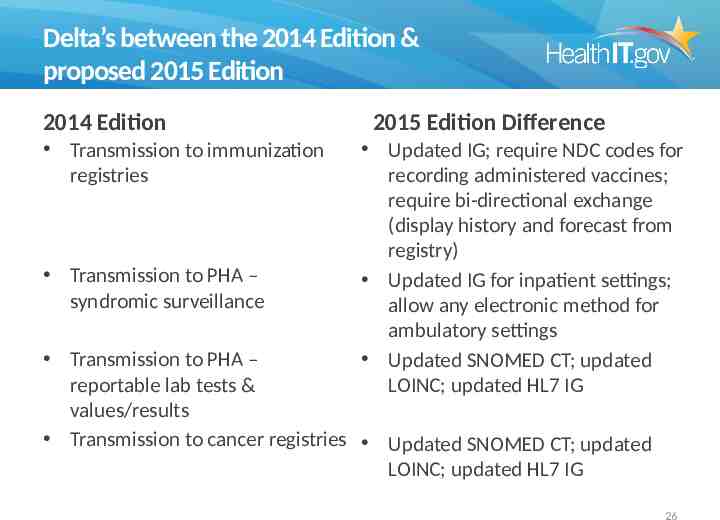
Delta’s between the 2014 Edition & proposed 2015 Edition 2014 Edition Transmission to immunization registries Transmission to PHA – syndromic surveillance 2015 Edition Difference Updated IG; require NDC codes for recording administered vaccines; require bi-directional exchange (display history and forecast from registry) Updated IG for inpatient settings; allow any electronic method for ambulatory settings Updated SNOMED CT; updated LOINC; updated HL7 IG Transmission to PHA – reportable lab tests & values/results Transmission to cancer registries Updated SNOMED CT; updated LOINC; updated HL7 IG 26
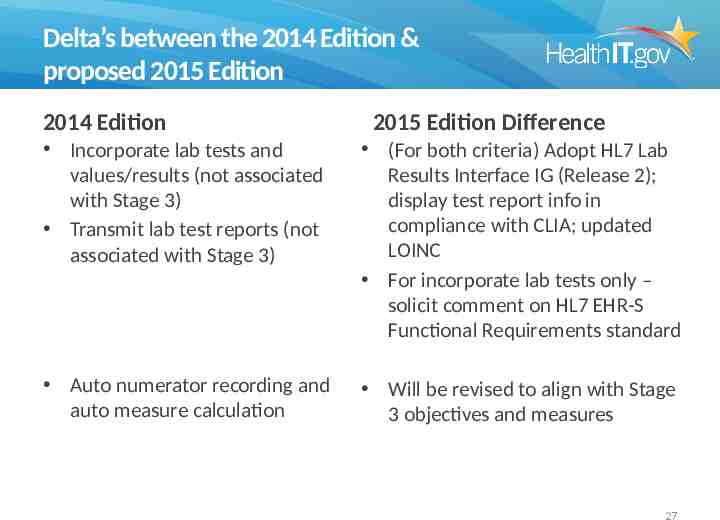
Delta’s between the 2014 Edition & proposed 2015 Edition 2014 Edition 2015 Edition Difference Incorporate lab tests and values/results (not associated with Stage 3) Transmit lab test reports (not associated with Stage 3) (For both criteria) Adopt HL7 Lab Results Interface IG (Release 2); display test report info in compliance with CLIA; updated LOINC For incorporate lab tests only – solicit comment on HL7 EHR-S Functional Requirements standard Auto numerator recording and auto measure calculation Will be revised to align with Stage 3 objectives and measures 27
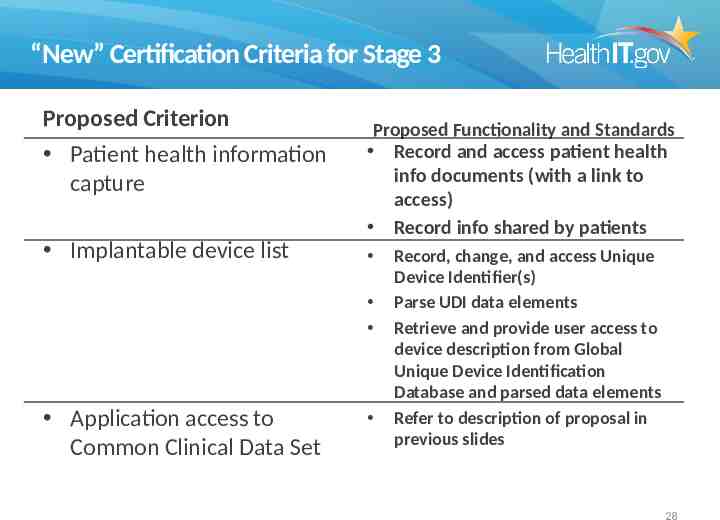
“New” Certification Criteria for Stage 3 Proposed Criterion Patient health information capture Implantable device list Proposed Functionality and Standards Record and access patient health info documents (with a link to access) Record info shared by patients Application access to Common Clinical Data Set Record, change, and access Unique Device Identifier(s) Parse UDI data elements Retrieve and provide user access to device description from Global Unique Device Identification Database and parsed data elements Refer to description of proposal in previous slides 28

“New” Certification Criteria for Stage 3 (continued) Proposed Criterion Consolidated CDA Creation Performance Proposed Functionality and Standards Create a data file in accordance with C-CDA Release 1.1 and Release 2.0 that matches a gold-standard, reference file Must be able to create document templates (CCD, Consultation Note, History and Physical, Progress Note, Care Plan, Transfer Summary, Referral Note, and (inpatient only) Discharge Summary) Must conform to vocabulary standards and value sets adopted in C-CDA Releases 1.1 29 and 2.0
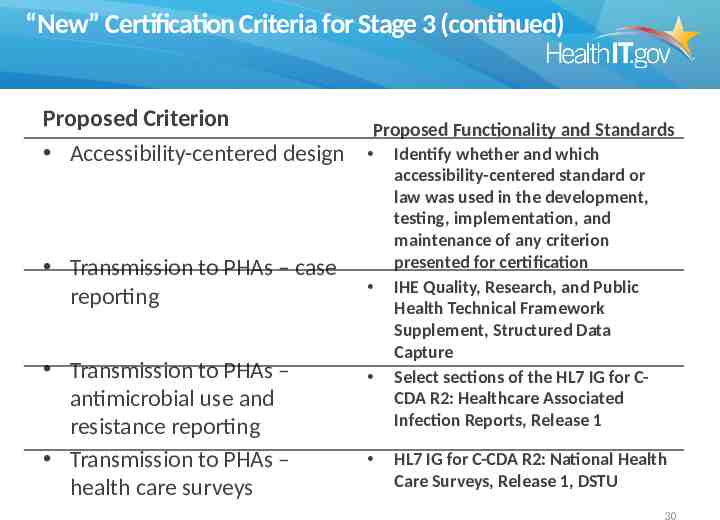
“New” Certification Criteria for Stage 3 (continued) Proposed Criterion Accessibility-centered design Transmission to PHAs – case reporting Transmission to PHAs – antimicrobial use and resistance reporting Transmission to PHAs – health care surveys Proposed Functionality and Standards Identify whether and which accessibility-centered standard or law was used in the development, testing, implementation, and maintenance of any criterion presented for certification IHE Quality, Research, and Public Health Technical Framework Supplement, Structured Data Capture Select sections of the HL7 IG for CCDA R2: Healthcare Associated Infection Reports, Release 1 HL7 IG for C-CDA R2: National Health Care Surveys, Release 1, DSTU 30

“New” Certification Criteria Not Tied to Stage 3 Proposed Criterion Social, psychological, and behavioral data Proposed Functionality and Standards Decision support – knowledge artifact Decision support – service Care Plan Enable user to record, change, access data (e.g., SOGI, education, depression, physical activity) using SNOMED CT and LOINC codes Enable user to send and receive CDS artifacts using HL7 V3: CDS Knowledge Artifacts Specification, Release 1.2, DSTU Enable a user to send and receive CDS using HL7 IG: Decision Support Service, Release 1.1, DSTU Enable a user to record, change, access, create and receive care plan info using the Care Plan document template in the C-CDA R2 31

“New” Certification Criteria Not Tied to Stage 3 (cont.) Proposed Criterion Data segmentation for privacy – send Data segmentation for privacy – receive Proposed Functionality and Standards Clinical quality measures filter Enable user to create summary record (in accordance with C-CDA Releases 1.1 and 2.0) tagged as restricted and subject to redisclosure restrictions in HL7 IG: DS4P, Release 1 Enable user to receive summary care record in accordance with HL7 IG: DS4P, Release 1 Apply document-level tagging and sequester document Allow user to view document Record data and filter CQM results (patient and aggregate levels) by provider and patient characteristics (e.g., TIN, NPI, patient age) 32

“New” Certification Criteria Not Tied to Stage 3 (cont.) Proposed Criterion Accessibility technology compatibility Healthcare provider directory - query request Proposed Functionality and Standards Healthcare provider directory - query response For any clinical (a), care coordination (b), or patient engagement (e) criterion presented for certification, demonstrate compatibility with at least one text-to-speech technology Request or respond to queries for individual, organizational, both individual and organizational providers, relationships between providers using IHE Healthcare Provide Directory, Trial Implementation Optional – process or make federated queries 33
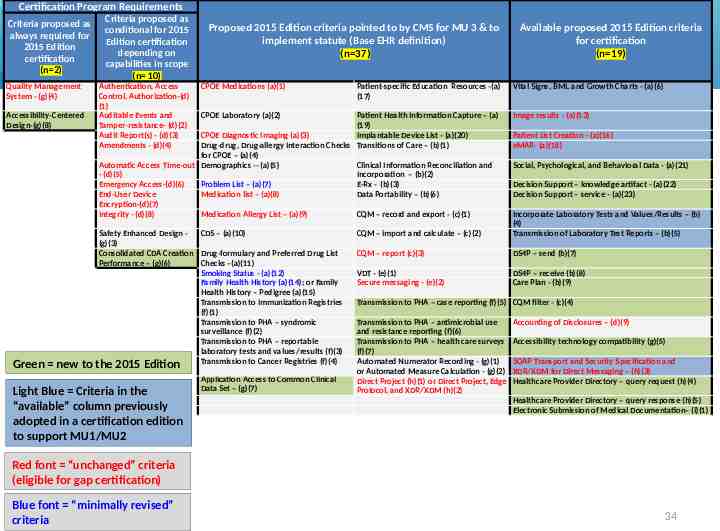
Certification Program Requirements Criteria proposed as always required for 2015 Edition certification (n 2) Quality Management System - (g)(4) Accessibility-Centered Design-(g)(8) Green new to Criteria proposed as conditional for 2015 Edition certification depending on capabilities in scope (n 10) Authentication, Access Control, Authorization-(d) (1) Auditable Events and Tamper-resistance- (d)(2) Audit Report(s) - (d)(3) Amendments - (d)(4) Proposed 2015 Edition criteria pointed to by CMS for MU 3 & to implement statute (Base EHR definition) (n 37) CPOE Medications (a)(1) Patient-specific Education Resources -(a) (17) CPOE Laboratory (a)(2) Patient Health Information Capture – (a) (19) CPOE Diagnostic Imaging (a)(3) Implantable Device List - (a)(20) Drug-drug, Drug-allergy Interaction Checks Transitions of Care – (b)(1) for CPOE – (a)(4) Automatic Access Time-out Demographics -- (a)(5) Clinical Information Reconciliation and - (d)(5) Incorporation – (b)(2) Emergency Access-(d)(6) Problem List – (a)(7) E-Rx - (b)(3) End-User Device Medication list – (a)(8) Data Portability – (b)(6) Encryption-(d)(7) Integrity - (d)(8) Medication Allergy List – (a)(9) CQM – record and export - (c)(1) Safety Enhanced Design CDS – (a)(10) (g)(3) Consolidated CDA Creation Drug-formulary and Preferred Drug List Performance – (g)(6) Checks –(a)(11) Smoking Status - (a)(12) Family Health History (a)(14); or Family Health History – Pedigree (a)(15) Transmission to Immunization Registries (f)(1) Transmission to PHA – syndromic surveillance (f)(2) Transmission to PHA – reportable laboratory tests and values/results (f)(3) Transmission to Cancer Registries (f)(4) the 2015 Edition Light Blue Criteria in the “available” column previously adopted in a certification edition to support MU1/MU2 Application Access to Common Clinical Data Set – (g)(7) Available proposed 2015 Edition criteria for certification (n 19) Vital Signs, BMI, and Growth Charts - (a)(6) Image results - (a)(13) Patient List Creation - (a)(16) eMAR- (a)(18) Social, Psychological, and Behavioral Data - (a)(21) Decision Support – knowledge artifact - (a)(22) Decision Support – service - (a)(23) CQM – import and calculate – (c)(2) Incorporate Laboratory Tests and Values/Results – (b) (4) Transmission of Laboratory Test Reports – (b)(5) CQM – report (c)(3) DS4P – send (b)(7) VDT - (e)(1) Secure messaging - (e)(2) DS4P – receive (b)(8) Care Plan - (b)(9) Transmission to PHA – case reporting (f)(5) CQM filter - (c)(4) Transmission to PHA – antimicrobial use and resistance reporting (f)(6) Transmission to PHA – health care surveys (f)(7) Automated Numerator Recording - (g)(1) or Automated Measure Calculation - (g)(2) Direct Project (h)(1) or Direct Project, Edge Protocol, and XDR/XDM (h)(2) Accounting of Disclosures – (d)(9) Accessibility technology compatibility (g)(5) SOAP Transport and Security Specification and XDR/XDM for Direct Messaging – (h)(3) Healthcare Provider Directory – query request (h)(4) Healthcare Provider Directory – query response (h)(5) Electronic Submission of Medical Documentation– (i)(1) Red font “unchanged” criteria (eligible for gap certification) Blue font “minimally revised” criteria 34

CMS Meaningful Use Stage 3 Proposed Rule Criteria 35

CMS MU Stage 3 Proposed Rule 36

CMS MU Stage 3 Proposed Rule Improvements Streamlining: Synchronizing on single stage and single reporting period Improving Outcomes: Ensuring providers caring for same patient are sharing info with one another Providing patients with easy access to health info Fostering data collection in sharable format across multiple health care organizations Supporting learning health system through sharing of common clinical dataset and expanding types of registries to which hospitals and providers can report Flexibility: Have option to report on Stage 3 criteria in 2017 Required to report on Stage 3 beginning in 2018 regardless of prior participation/stage of meaningful use 37

CMS MU Stage 3 Proposed Objectives Stage 3 Proposed Objectives Protect Electronic Health Information Electronic Prescribing (eRx) Clinical Decision Support Computerized Provider Order Entry (CPOE) Patient Electronic Access to Health Information Coordination of Care through Patient Engagement Health Information Exchange Public Health Reporting * Modified and Expanded from Stage 2 38
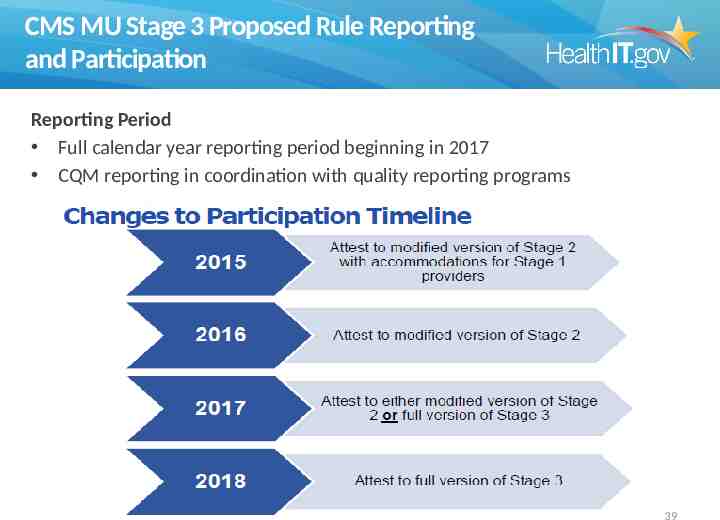
CMS MU Stage 3 Proposed Rule Reporting and Participation Reporting Period Full calendar year reporting period beginning in 2017 CQM reporting in coordination with quality reporting programs 39

Objectives Discuss the movement of Meaningful Use from Stage 2 to Stage 3 Discuss recent Congressional legislation and other proposed changes to quality measurement and payment The role of interoperability in quality and payment moving forward Learn about the current standards strategy for health IT in the US government moving towards this new vision from HHS 40

Improving Medicare Post-Acute Care Transformation (IMPACT) Act of 2014 Bi-partisan bill introduced in March, U.S. House & Senate. Requires: – standardized patient assessment data to create uniformity and compare quality across PAC settings; and – standardized and interoperable patient assessment data to allow for the exchange of such data among PAC and other providers to facilitate: Quality care and improved outcomes Improve discharge planning Facilitate care coordination across the care continuum

Required: Standardized Assessment Data IMPACT Act added new section 1899(B) to Title XVIII of the Social Security Act (SSA) Post-Acute Care (PAC) providers must report: – Standardized assessment data – Data on quality measures – Data on resource use and other measures The data must be standardized and interoperable to allow for the: – Exchange of data using common standards and definitions – Facilitation of care coordination – Improvement of Medicare beneficiary outcomes PAC assessment instruments must be modified to: – Enable the submission of standardized data – Compare data across all applicable providers

IMPACT ACT: Standardized Patient Assessment Data – Providers must submit standardized assessment data through PAC assessment instruments under applicable reporting provisions – The data must be submitted with respect to admission and discharge for each patient, or more frequently as required Data categories: – Functional status – Cognitive function and mental status – Special services, treatments, and interventions – Medical conditions and co-morbidities Use of Standardized – Impairments Assessment Data: HHAs: no later than January – Other categories required by the Secretary 1, 2019 SNFs, IRFs, and LTCHs: no later than October 1, 2018

IMPACT ACT: Quality Measure Domains Requirements: – Measures must be uniform/standardized across the 4 settings – Measures will be risk adjusted, as determined appropriate by the Secretary Domains: – Functional status, cognitive function, and changes in function and cognitive function – Skin integrity and changes in skin integrity – Medication reconciliation – Incidence of major falls – Communicating the existence of and providing for the transfer of health information and care preferences

IMPACT Act: Resource Use and Other Measures Resource use and other measures will be specified for reporting, which may include standardized assessment data in addition to claims data. Resource use and other measure domains include: – Total estimated Medicare spending per beneficiary – Discharge to community – Measures to reflect all-condition risk-adjusted potentially preventable hospital readmission rates
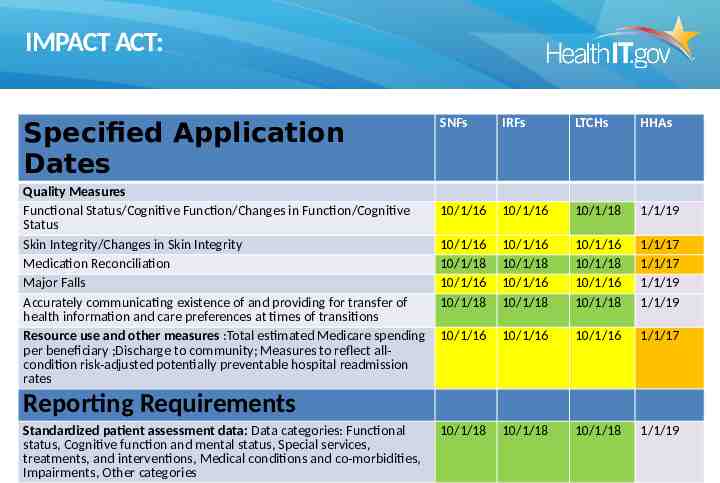
IMPACT ACT: Specified Application Dates Quality Measures Functional Status/Cognitive Function/Changes in Function/Cognitive Status Skin Integrity/Changes in Skin Integrity Medication Reconciliation Major Falls Accurately communicating existence of and providing for transfer of health information and care preferences at times of transitions Resource use and other measures :Total estimated Medicare spending per beneficiary ;Discharge to community; Measures to reflect allcondition risk-adjusted potentially preventable hospital readmission rates SNFs IRFs LTCHs HHAs 10/1/16 10/1/16 10/1/18 1/1/19 10/1/16 10/1/18 10/1/16 10/1/18 10/1/16 10/1/18 10/1/16 10/1/18 10/1/16 10/1/18 10/1/16 10/1/18 1/1/17 1/1/17 1/1/19 1/1/19 10/1/16 10/1/16 10/1/16 1/1/17 10/1/18 10/1/18 10/1/18 1/1/19 Reporting Requirements Standardized patient assessment data: Data categories: Functional status, Cognitive function and mental status, Special services, treatments, and interventions, Medical conditions and co-morbidities, Impairments, Other categories
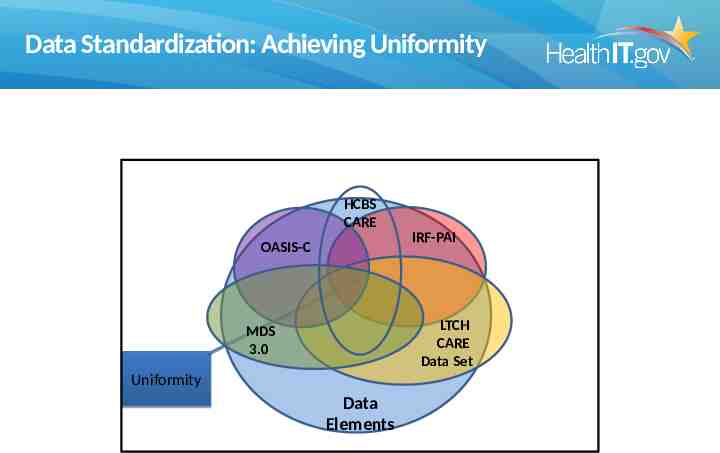
Data Standardization: Achieving Uniformity HCBS CARE OASIS-C IRF-PAI LTCH CARE Data Set MDS 3.0 Uniformity Data Elements

IMPACT Act: Measurement Implementation Phases Selection of Quality Measures and Resource Use and Other Measures (1)Measurement Implementation Phases (A)Initial Implementation Phase (i) measure specification (ii) data collection (B) Second Implementation Phase – feedback reports to PAC providers (C) Third Implementation Phase – public reporting of PAC providers' performance (2) Consensus-based Entity (3) Treatment of Application of Pre-Rulemaking Process
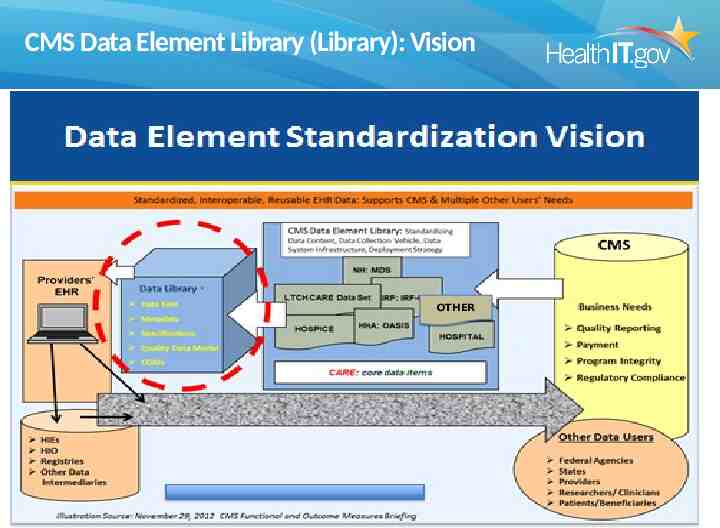
CMS Data Element Library (Library): Vision OTHER

Medicare Access & CHIP Reauthorization Act of 2015: Shifting from FFS to P4P The Medicare Access & CHIP Reauthorization Act of 2015 (MACRA) will achieve the following: Temporarily extend (until September 30, 2017) the Children’s Health Insurance Program (CHIP) Stabilize annual physician fee schedule payment until 2019 Adjustments to the amounts paid to individual providers via one of two mechanisms: (Beginning in 2019) Alternative Payment Model (APM) 2019: Merit-Based Incentive Payment System (MIPS) begins Establish a new program for technical assistance provision for MIPS-eligible providers in small practices (15 or fewer professionals) who serve in rural & underserved communities MANY of the details have yet to be determined and there are several areas where feedback will be sought from the public and health care community. March 24, 2015 March 26, 2015 April 14, 2015 Office of the National April 16, 2015 50
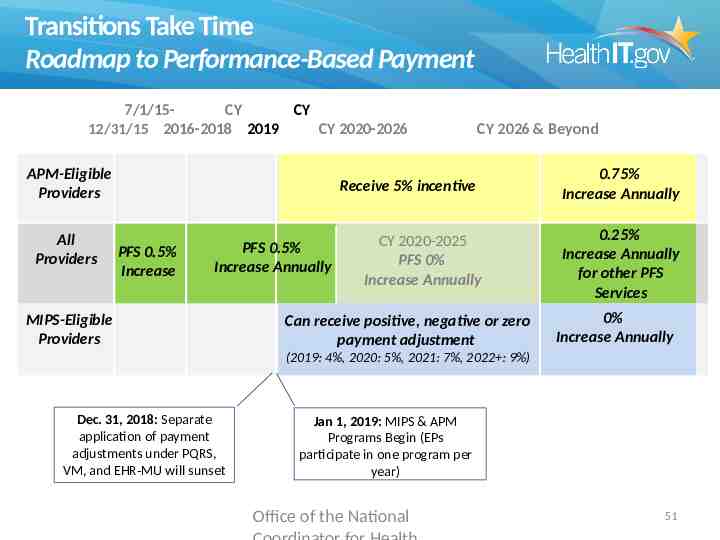
Transitions Take Time Roadmap to Performance-Based Payment 7/1/15CY CY 12/31/15 2016-2018 2019 CY 2020-2026 APM-Eligible Providers All Providers CY 2026 & Beyond Receive 5% incentive PFS 0.5% Increase PFS 0.5% Increase Annually MIPS-Eligible Providers CY 2020-2025 PFS 0% Increase Annually Can receive positive, negative or zero payment adjustment 0.75% Increase Annually 0.25% Increase Annually for other PFS Services 0% Increase Annually (2019: 4%, 2020: 5%, 2021: 7%, 2022 : 9%) Dec. 31, 2018: Separate application of payment adjustments under PQRS, VM, and EHR-MU will sunset Jan 1, 2019: MIPS & APM Programs Begin (EPs participate in one program per year) Office of the National 51

Appropriate Use Criteria for Advanced Diagnostic Imaging Services Section 218(b) of the PAMA amended Title XVIII of the Act, to establish a program to promote the use of appropriate use criteria (AUC) for advanced imaging services. The legislation requires in 2018 that every claim for advanced radiologic studies would include both evidence that the user had utilized some form of approved clinical decision support that supported “appropriate use” of the advanced radiologic study and evidence of whether the user adhered to that advice 52

Appropriate Use Criteria for Advanced Diagnostic Imaging Services The goal of the PAMA legislation is to curb the ordering of unnecessary advanced radiologic studies, which make up billions of dollars of Medicare and Medicaid spending “Advanced radiologic studies” includes CT, MRI, fMRI, SPECT, PET and other nuclear studies but not traditional X-rays 53

Appropriate Use Criteria for Advanced Diagnostic Imaging Services CMS is currently establishing criteria for clinical “appropriate use” In 2016 they will establish how users can technically demonstrate the use of “appropriate use” In 2019 Congress mandated that CMS start to require preauthorization of radiologic studies for entities and individuals found to be regularly ordering studies deemed “inappropriate” according to the established “appropriate use” criteria 54

Electronic Specifications for the Core Clinical Data Elements for Risk Adjustment of Hospital-Level Outcome Measures Yale University did an analysis of thousands of records for hospitalizations of Medicare patients to determine which characteristics predicted higher risk of mortality across all hospitalizations They determined the first measurement of 22 lab and vital sign values as well as the demographics age and gender were the most predictive and were amenable to risk adjustment by CMS CMS seeks risk adjustment to determine whether some hospital case mixes include patients who are more sick than others and therefore would expect worse outcomes A similar analysis was performed specifically for ischemic stroke patients and was also released
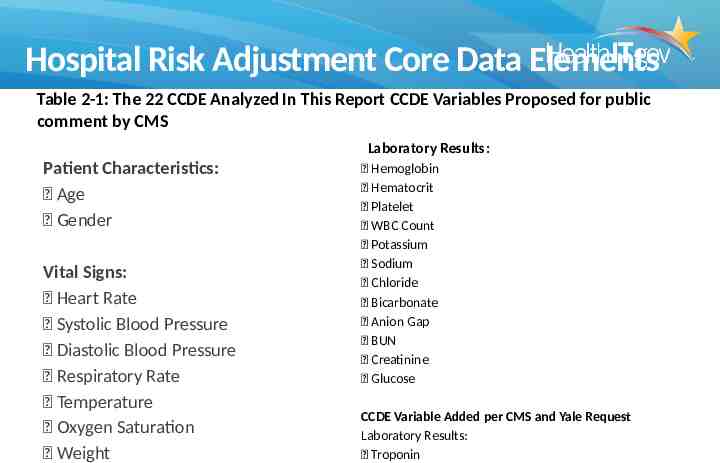
Hospital Risk Adjustment Core Data Elements Table 2-1: The 22 CCDE Analyzed In This Report CCDE Variables Proposed for public comment by CMS Laboratory Results: Patient Characteristics: Age Gender Vital Signs: Heart Rate Systolic Blood Pressure Diastolic Blood Pressure Respiratory Rate Temperature Oxygen Saturation Weight Hemoglobin Hematocrit Platelet WBC Count Potassium Sodium Chloride Bicarbonate Anion Gap BUN Creatinine Glucose CCDE Variable Added per CMS and Yale Request Laboratory Results: Troponin

Hospital Risk Adjustment Core Data Elements How would the Core Common Data Elements for hospitals be implemented? In 2015, CMS included a proposal to use QDMbased HQMF to specify the first of each of the data elements in the list with the expectation that users would return a QRDA-I file Initially this is expected to be submitted for all Medicare hospitalizations although CMS’ authority would allow them to collect on all payers

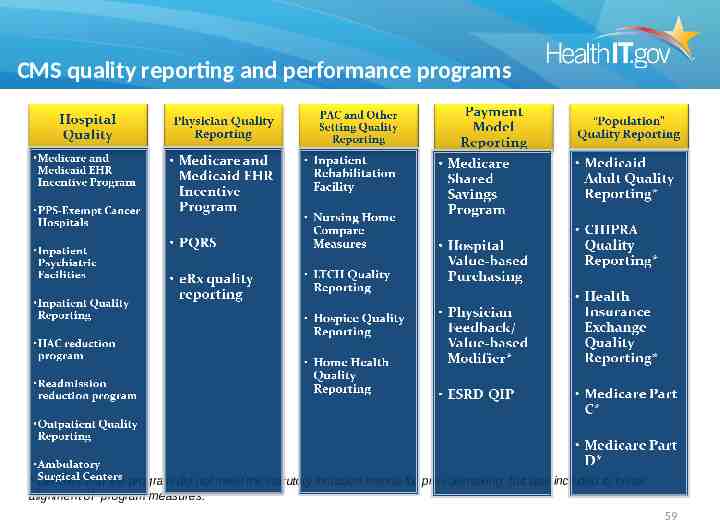
MACRA: Medicare Access and CHIP Reauthorization Act of 2015 Beginning in 2019, all current Medicare payment, including incentive programs, will be combined into one Merit-Based Incentive Payment System (MIPS), replacing all Medicare reimbursement for eligible professionals. The MIPS program will use four performance measures to determine reimbursement, which will begin in 2019: Quality; Resource use; Clinical practice improvement activities; and Meaningful use of certified EHR technology. Privacy and security including HIPAA are also requirements and failure to adhere to required standards results in penalties 58
CMS quality reporting and performance programs * Denotes that the program did not meet the statutory inclusion criteria for pre-rulemaking, but was included to foster alignment of program measures. 59
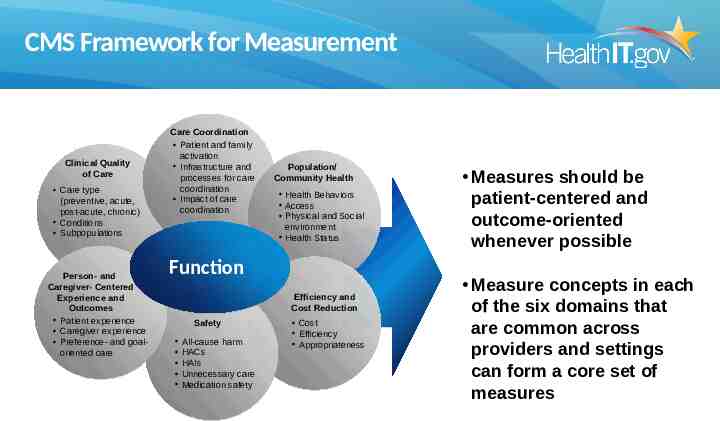
CMS Framework for Measurement Clinical Quality of Care Care type (preventive, acute, post-acute, chronic) Conditions Subpopulations Person- and Caregiver- Centered Experience and Outcomes Patient experience Caregiver experience Preference- and goaloriented care Care Coordination Patient and family activation Infrastructure and processes for care coordination Impact of care coordination Population/ Community Health Health Behaviors Access Physical and Social environment Health Status Function Efficiency and Cost Reduction Safety All-cause harm HACs HAIs Unnecessary care Medication safety Cost Efficiency Appropriateness Measures should be patient-centered and outcome-oriented whenever possible Measure concepts in each of the six domains that are common across providers and settings can form a core set of measures
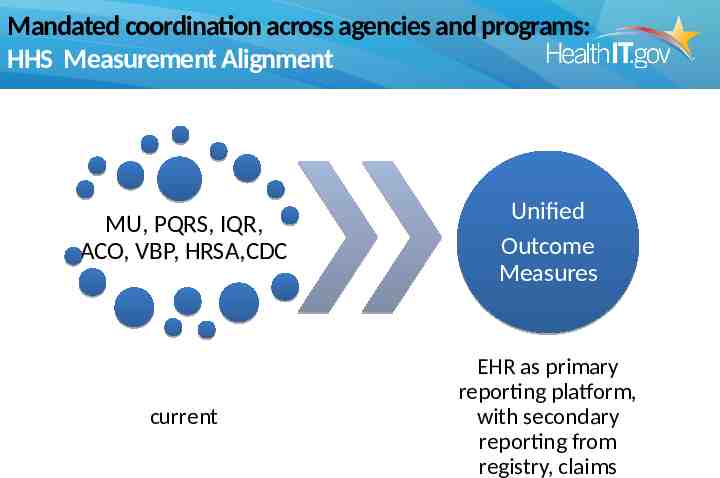
Mandated coordination across agencies and programs: HHS Measurement Alignment MU, PQRS, IQR, ACO, VBP, HRSA,CDC Unified Outcome Measures current EHR as primary reporting platform, with secondary reporting from registry, claims

Medicare Access & CHIP Reauthorization Act of 2015: Shifting from FFS to P4P The Medicare Access & CHIP Reauthorization Act of 2015 (MACRA) will achieve the following: Temporarily extend (until September 30, 2017) the Children’s Health Insurance Program (CHIP) Stabilize annual physician fee schedule payment until 2019 Adjustments to the amounts paid to individual providers via one of two mechanisms: (Beginning in 2019) Alternative Payment Model (APM) 2019: Merit-Based Incentive Payment System (MIPS) begins Establish a new program for technical assistance provision for MIPS-eligible providers in small practices (15 or fewer professionals) who serve in rural & underserved communities MANY of the details have yet to be determined and there are several areas where feedback will be sought from the public and health care community. March 24, 2015 March 26, 2015 April 14, 2015 Office of the National April 16, 2015 62
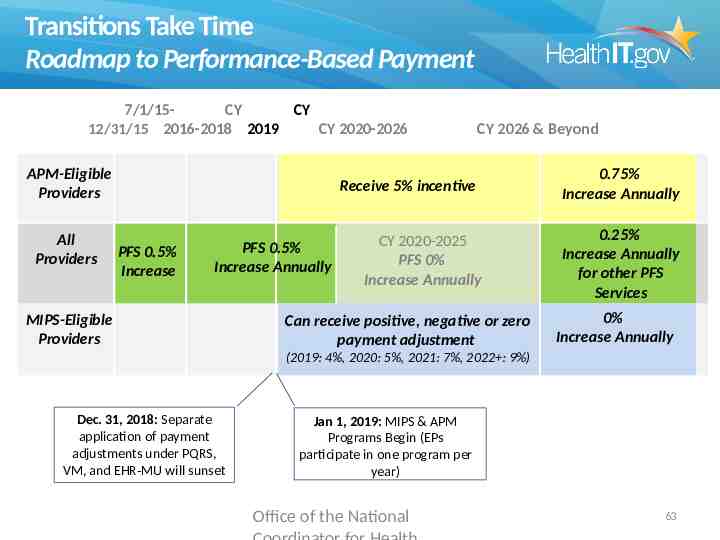
Transitions Take Time Roadmap to Performance-Based Payment 7/1/15CY CY 12/31/15 2016-2018 2019 CY 2020-2026 APM-Eligible Providers All Providers CY 2026 & Beyond Receive 5% incentive PFS 0.5% Increase PFS 0.5% Increase Annually MIPS-Eligible Providers CY 2020-2025 PFS 0% Increase Annually Can receive positive, negative or zero payment adjustment 0.75% Increase Annually 0.25% Increase Annually for other PFS Services 0% Increase Annually (2019: 4%, 2020: 5%, 2021: 7%, 2022 : 9%) Dec. 31, 2018: Separate application of payment adjustments under PQRS, VM, and EHR-MU will sunset Jan 1, 2019: MIPS & APM Programs Begin (EPs participate in one program per year) Office of the National 63
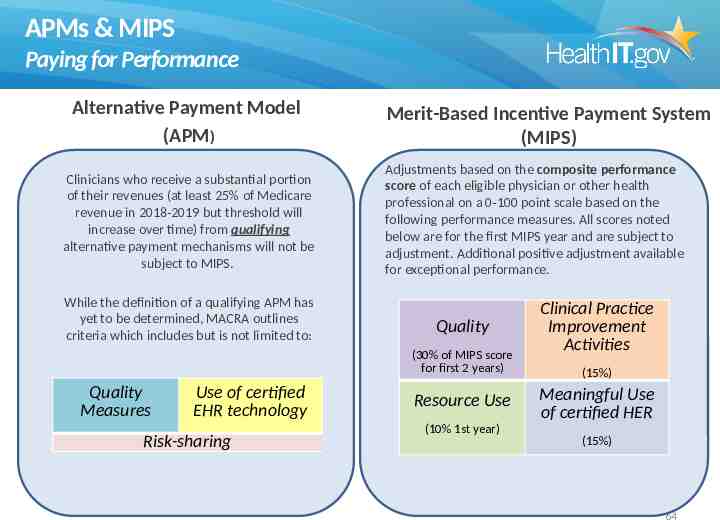
APMs & MIPS Paying for Performance Alternative Payment Model (APM) Clinicians who receive a substantial portion of their revenues (at least 25% of Medicare revenue in 2018-2019 but threshold will increase over time) from qualifying alternative payment mechanisms will not be subject to MIPS. While the definition of a qualifying APM has yet to be determined, MACRA outlines criteria which includes but is not limited to: Merit-Based Incentive Payment System (MIPS) Adjustments based on the composite performance score of each eligible physician or other health professional on a 0-100 point scale based on the following performance measures. All scores noted below are for the first MIPS year and are subject to adjustment. Additional positive adjustment available for exceptional performance. Quality (30% of MIPS score for first 2 years) Quality Measures Use of certified EHR technology Risk-sharing Resource Use (10% 1st year) Clinical Practice Improvement Activities (15%) Meaningful Use of certified HER (15%) 64
MIPS-Eligible Professionals (EP) Notable Dates July 1, 2017 CMS must make available timely confidential feedback reports to each MIPS EP 2017 2018 2019 Office of the National 2020 2021 65

MIPS-Eligible Professionals (EP) Notable Dates July 1, 2017 CMS must make available timely confidential feedback reports to each MIPS EP 2017 2018 2019 2020 2021 July 1, 2018 CMS must make available to each MIPS EP information about items and services furnished to the EP’s patients by other providers and suppliers for which payment is made under Medicare Office of the National 66

MIPS-Eligible Professionals (EP) Notable Dates Qualifying EPs 2019-20 July 1, 2017 CMS must make available timely confidential feedback reports to each MIPS EP 2017 2018 Physicians PAs Certified RN Anesthetists NPs Clinical Nurse Specialists Groups that include such professionals 2019 2020 2021 July 1, 2018 CMS must make available to each MIPS EP information about items and services furnished to the EP’s patients by other providers and suppliers for which payment is made under Medicare Office of the National 67

MIPS-Eligible Professionals (EP) Notable Dates Qualifying EPs 2019-20 July 1, 2017 CMS must make available timely confidential feedback reports to each MIPS EP 2017 2018 Physicians PAs Certified RN Anesthetists NPs Clinical Nurse Specialists Groups that include such professionals 2019 July 1, 2018 CMS must make available to each MIPS EP information about items and services furnished to the EP’s patients by other providers and suppliers for which payment is made under Medicare Office of the National 2020 2021 2021 & Onward Secretary can add EPs to MIPS 68

MIPS-Eligible Professionals (EP) Notable Dates Qualifying EPs 2019-20 July 1, 2017 CMS must make available timely confidential feedback reports to each MIPS EP 2017 2018 Physicians PAs Certified RN Anesthetists NPs Clinical Nurse Specialists Groups that include such professionals 2019 July 1, 2018 CMS must make available to each MIPS EP information about items and services furnished to the EP’s patients by other providers and suppliers for which payment is made under Medicare Office of the National 2020 2021 2021 & Onward Secretary can add EPs to MIPS 69

MIPS Performance Feedback Sharing Performance Info with Providers & General Public MIPS – CMS must make available timely (“such as quarterly”) confidential feedback reports to each MIPS EP starting July 1, 2017 – Beginning July 1, 2018, CMS must make available to each MIPS EP information about items and services furnished to the EP’s patients by other providers and suppliers for which payment is made under Medicare – Information about the performance of MIPS EPs must be made available on Physician Compare 01/07/2023 70

MACRA: Medicare Access and CHIP Reauthorization Act of 2015 Failing to perform to the program minimums results in payment penalties: 2019- 4% maximum penalty 2020- 5% maximum penalty 2021- 7% maximum penalty 2022- 9% maximum penalty Eligible professionals with higher performance scores receive an incentive up to three times the annual cap for negative payment adjustments Nationwide interoperability is a requirement by December 31, 2018 71
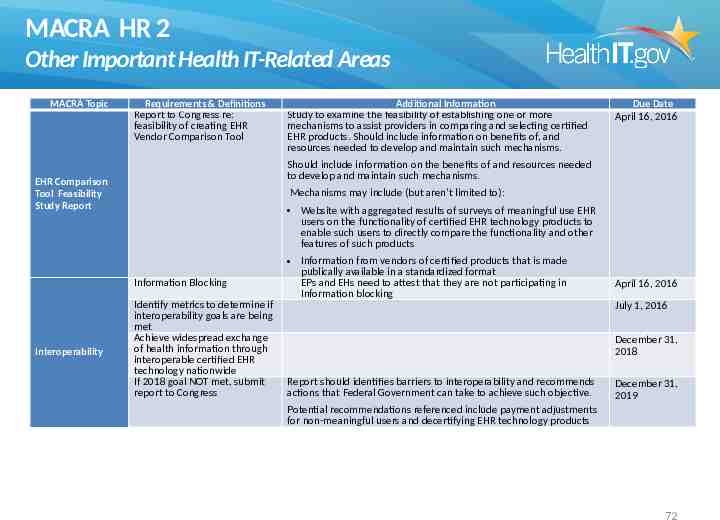
MACRA HR 2 Other Important Health IT-Related Areas MACRA Topic Requirements & Definitions Report to Congress re: feasibility of creating EHR Vendor Comparison Tool Due Date April 16, 2016 Should include information on the benefits of and resources needed to develop and maintain such mechanisms. EHR Comparison Tool Feasibility Study Report Mechanisms may include (but aren’t limited to): Website with aggregated results of surveys of meaningful use EHR users on the functionality of certified EHR technology products to enable such users to directly compare the functionality and other features of such products Information Blocking Interoperability Additional Information Study to examine the feasibility of establishing one or more mechanisms to assist providers in comparing and selecting certified EHR products. Should include information on benefits of, and resources needed to develop and maintain such mechanisms. Identify metrics to determine if interoperability goals are being met Achieve widespread exchange of health information through interoperable certified EHR technology nationwide If 2018 goal NOT met, submit report to Congress Information from vendors of certified products that is made publically available in a standardized format EPs and EHs need to attest that they are not participating in Information blocking April 16, 2016 July 1, 2016 December 31, 2018 Report should identifies barriers to interoperability and recommends actions that Federal Government can take to achieve such objective. December 31, 2019 Potential recommendations referenced include payment adjustments for non-meaningful users and decertifying EHR technology products 72

Objectives Discuss the movement of Meaningful Use from Stage 2 to Stage 3 Discuss recent Congressional legislation and other proposed changes to quality measurement and payment The role of interoperability in quality and payment moving forward Learn about the current standards strategy for health IT in the US government moving towards this new vision from HHS 73

2015 Interoperability Standards Advisory: http://www.healthit.gov/standards-advisory Contains Proposed Standards For: Vocabulary/Terminology Content and Structure Transport Services And an open process for annual updates 74
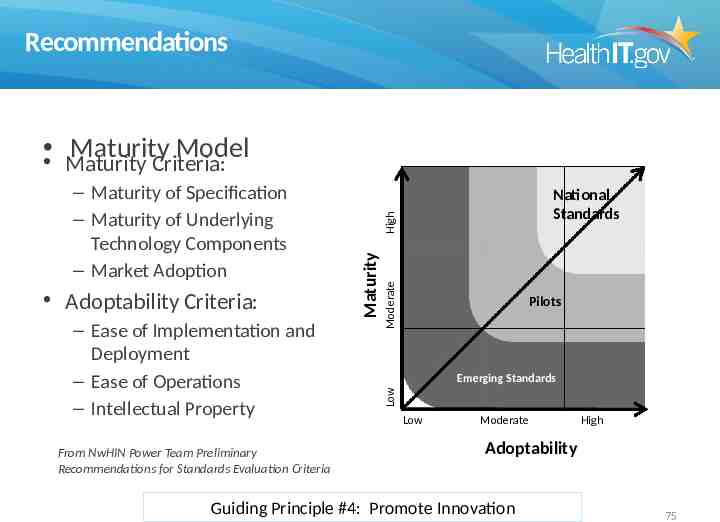
Recommendations Maturity Model Maturity Criteria: From NwHIN Power Team Preliminary Recommendations for Standards Evaluation Criteria High Moderate – Ease of Implementation and Deployment – Ease of Operations – Intellectual Property National Standards Pilots Emerging Standards Low Adoptability Criteria: Maturity – Maturity of Specification – Maturity of Underlying Technology Components – Market Adoption Low Moderate High Adoptability Guiding Principle #4: Promote Innovation 75

Update Process/Scope ISA Annual Update Process Security standards create a challenge due to the standards dynamic nature to update in case of a compromise, generating a need to raise awareness around emergent updates in such situations. Stability of a standard needs to be intertwined with promoting innovation to meet healthcare goals as it may vary from deployment to deployment but the goal should be to maintain consistency. ISA Scope The ISA scope should include a Use Case layer near the beginning and a column to the right for each of the use cases the standard is intended to satisfy. Cross walking between use cases and functionalities and explore the ability to tie functionality to use cases The ISA scope should point to all the preconditions, dependencies needed to facilitate interoperability or it should have a disclaimer that not all the constraints have been defined. 76

Future Changes or Improvements to ISA How does ONC ensure ISA is relevant for intended stakeholders? Relate standards to real-world, value-added outcomes, clinical processes, and business functions to ensure they address real world requirements What are the top priorities for ISA in 2016? Adjust organization as recommended, include implementation guidance Characterize Best Available standards in terms of maturity, testing, adoption, preconditions and dependencies, and ability to meet goals Identify a set of real-world, value-added outcomes and business functions to which standards are subordinate Do not rush to identify standards if a Best Available is not evident, but do not stifle innovation by requiring full maturity in all cases What are the top priorities for ISA in future (post 2016) releases? Continuously update maturity and adjust outcomes and functions as necessary Expand to include information on adoption/commitment to emerging standards, vendor roadmaps Broaden to include the full spectrum of healthcare needs 77
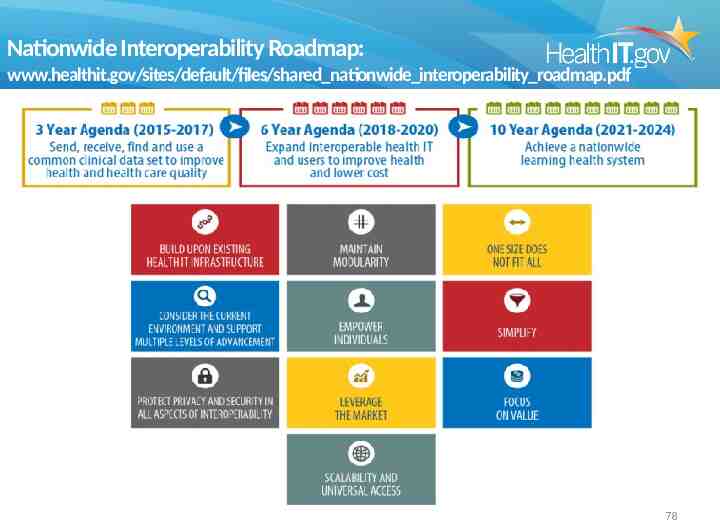
Nationwide Interoperability Roadmap: www.healthit.gov/sites/default/files/shared nationwide interoperability roadmap.pdf 78
79

Objectives Discuss the movement of Meaningful Use from Stage 2 to Stage 3 Discuss recent Congressional legislation and other proposed changes to quality measurement and payment The role of interoperability in quality and payment moving forward Learn about the current standards strategy for health IT in the US government moving towards this new vision from HHS 80

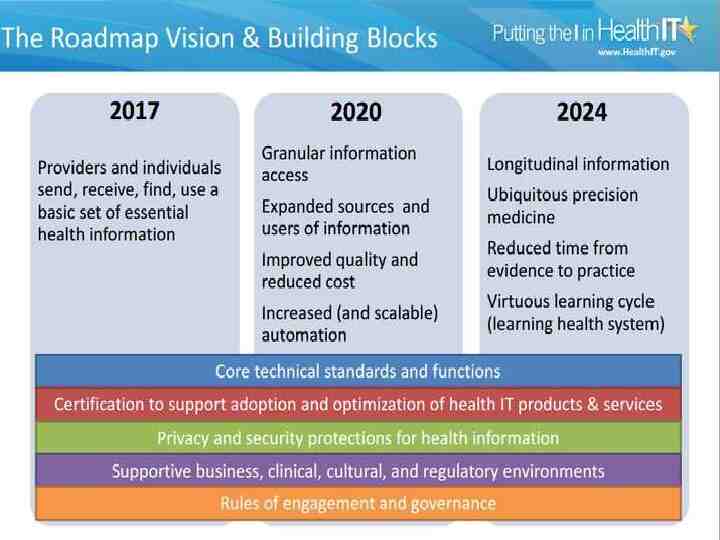
The Future Vision: Seamless, evidence-based, interoperable health data any time, everywhere Common data elements with common definitions Standards that promote interoperability and secure exchange Use of APIs to facilitate more agile systems and data integration Modular certification and testing that ensures universal adherence to both Increasing integration of patient-generated, sensor-based, and mobile data with sophisticated, informed data analytics to enable timely, personalized optimal health 81
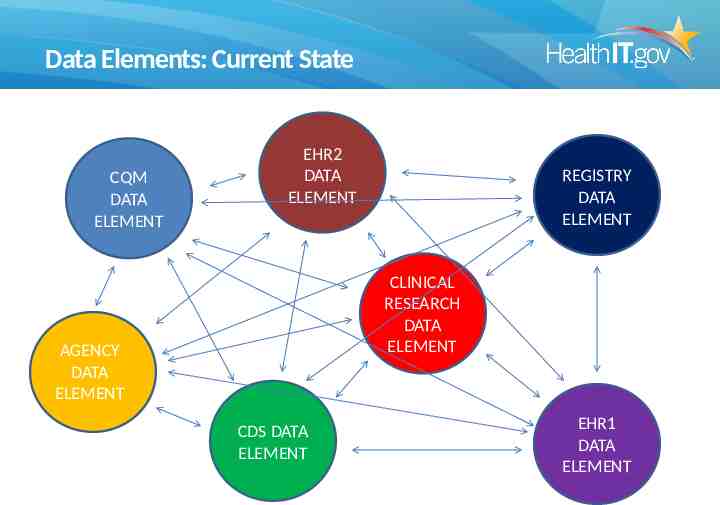
Data Elements: Current State CQM DATA ELEMENT EHR2 DATA ELEMENT REGISTRY DATA ELEMENT CLINICAL RESEARCH DATA ELEMENT AGENCY DATA ELEMENT CDS DATA ELEMENT EHR1 DATA ELEMENT
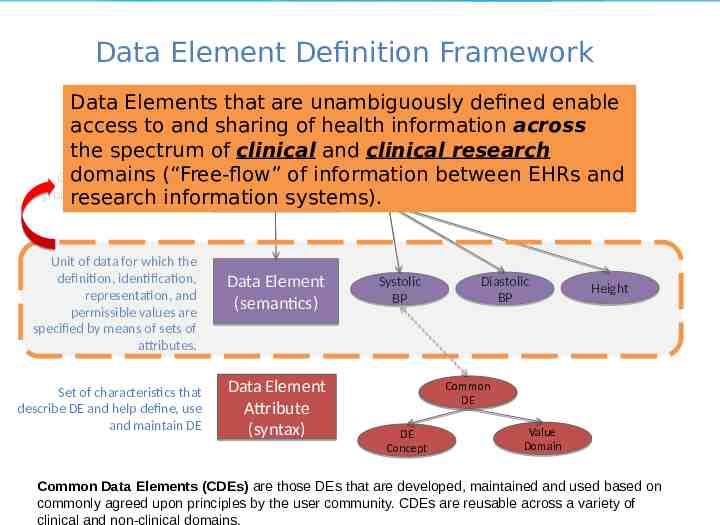
Data Element Definition Framework Data Elements that are unambiguously defined enable access to and sharing of health information across Medications Allergies the spectrum of clinical and clinical research domains (“Free-flow” of information between EHRsLaband Object containing logical Problems Vitals Data Set Results groupings of data elements research information systems). Unit of data for which the definition, identification, representation, and permissible values are specified by means of sets of attributes. Set of characteristics that describe DE and help define, use and maintain DE Data Element (semantics) Data Element Attribute (syntax) Systolic BP Diastolic BP Height Common DE DE Concept Value Domain Common Data Elements (CDEs) are those DEs that are developed, maintained and used based on commonly agreed upon principles by the user community. CDEs are reusable across a variety of clinical and non-clinical domains.
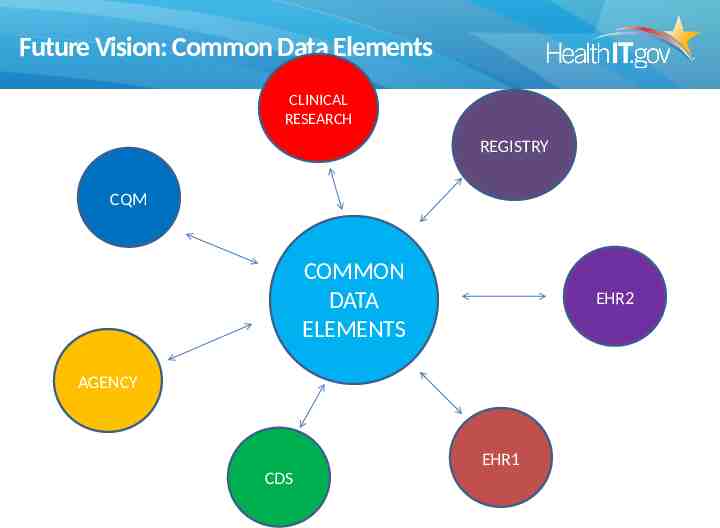
Future Vision: Common Data Elements CLINICAL RESEARCH REGISTRY CQM COMMON DATA ELEMENTS EHR2 AGENCY EHR1 CDS
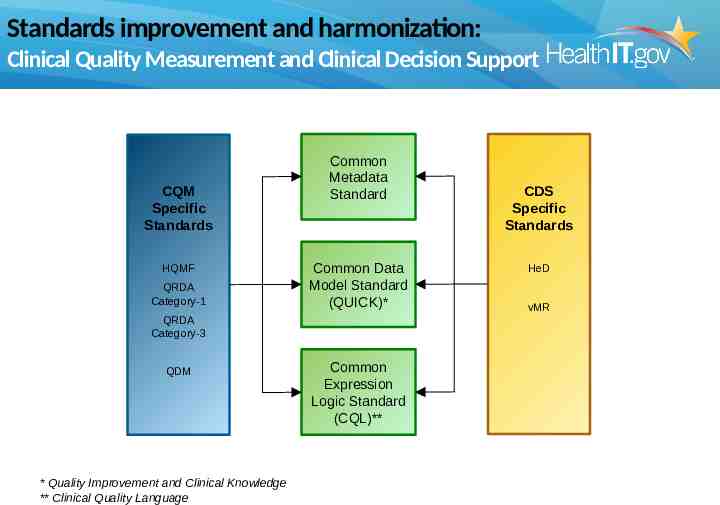
Standards improvement and harmonization: Clinical Quality Measurement and Clinical Decision Support CQM Specific Standards HQMF QRDA Category-1 Common Metadata Standard Common Data Model Standard (QUICK)* QRDA Category-3 QDM * Quality Improvement and Clinical Knowledge ** Clinical Quality Language Common Expression Logic Standard (CQL)** CDS Specific Standards HeD vMR
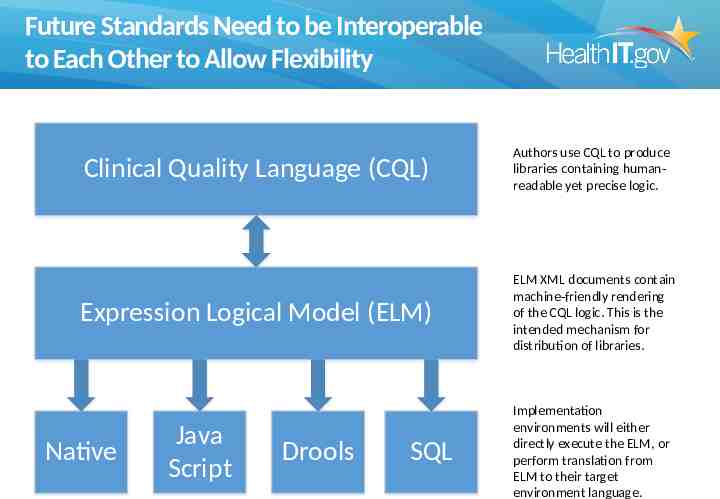
Future Standards Need to be Interoperable to Each Other to Allow Flexibility Clinical Quality Language (CQL) Authors use CQL to produce libraries containing humanreadable yet precise logic. Expression Logical Model (ELM) ELM XML documents contain machine-friendly rendering of the CQL logic. This is the intended mechanism for distribution of libraries. Native Java Script Drools SQL Implementation environments will either directly execute the ELM, or perform translation from ELM to their target environment language.
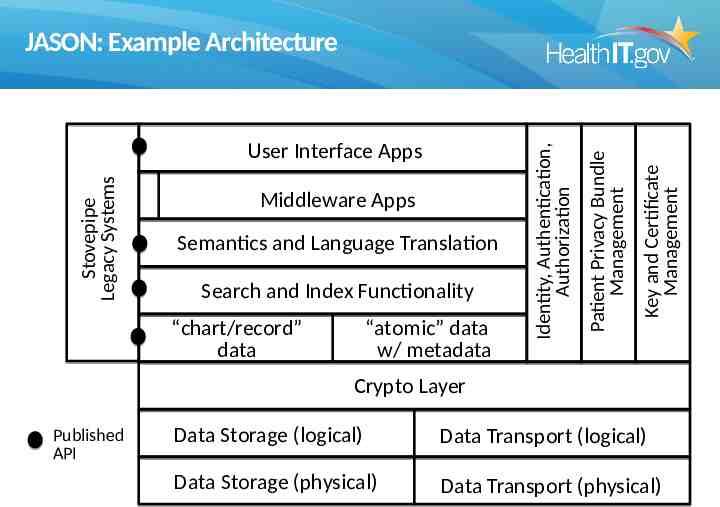
Semantics and Language Translation Search and Index Functionality “chart/record” data “atomic” data w/ metadata Key and Certificate Management Middleware Apps Patient Privacy Bundle Management Stovepipe Legacy Systems User Interface Apps Identity, Authentication, Authorization JASON: Example Architecture Crypto Layer Published API Data Storage (logical) Data Transport (logical) Data Storage (physical) Data Transport (physical)
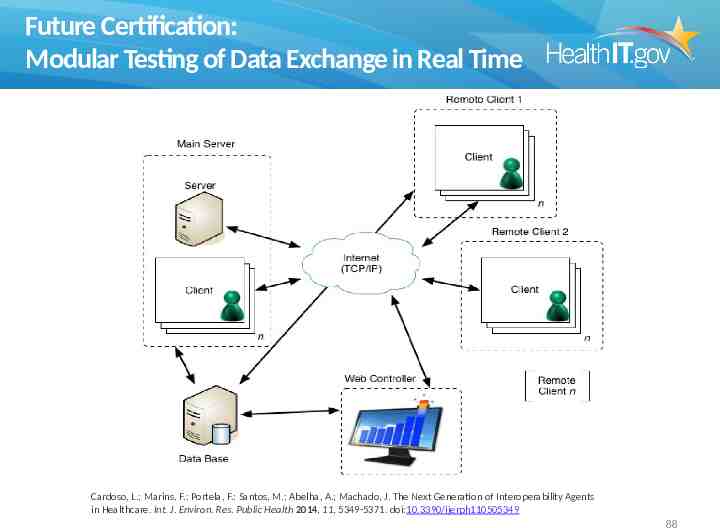
Future Certification: Modular Testing of Data Exchange in Real Time Cardoso, L.; Marins, F.; Portela, F.; Santos, M.; Abelha, A.; Machado, J. The Next Generation of Interoperability Agents in Healthcare. Int. J. Environ. Res. Public Health 2014, 11, 5349-5371. doi:10.3390/ijerph110505349 88

The Future of Health and Personal Health Data 89

The Electronic Clinical Quality Improvement (eCQI) Resource Center What is the eCQI Resource Center? The Resource Center is designed to act as a central hub for storing and collating resources surrounding the eCQMs and CDS standards, measures, tools, and guidance. It is cosponsored by CMS and ONC It will continue to add functionality and additional related content over time We welcome your feedback!

The Electronic Clinical Quality Improvement (eCQI) Resource Center Summer 2015 updates: Quick top-level navigation bar QDM and QRDA page enhancements Engage with eCQI page eCQI Implementers’ Corner eCQM 101 & 102 & resources Measure detail enhanced Measure scoring Type Improvement notation and guidance Improved Tools and Resources Page
The Electronic Clinical Quality Improvement (eCQI) Resource Center Summer 2015 updates: Kaizen Info and summary Added content tagging: User type User level eCQI Topic eCQI Function Content author Updated Measure Logic Document IE9 support Measure Logic Flows Visit at https://ecqi.healthit.gov The eCQI Resource Center is brought to you by the Centers for Medicare & Medicaid Services (CMS) and the Office of the National Coordinator for Health Information Technology (ONC)

The Future Vision: Seamless, evidence-based, interoperable health data any time, everywhere Common data elements with common definitions Standards that promote interoperability and secure exchange Use of APIs to facilitate more agile systems and data integration Modular certification and testing that ensures universal adherence to both Increasing integration of patient-generated, sensor-based, and mobile data with sophisticated, informed data analytics to enable timely, personalized optimal health 93

Questions and Feedback Julia Skapik, MD, MPH Office of Standards & Technology, Office of the National Coordinator for Health IT [email protected] 94
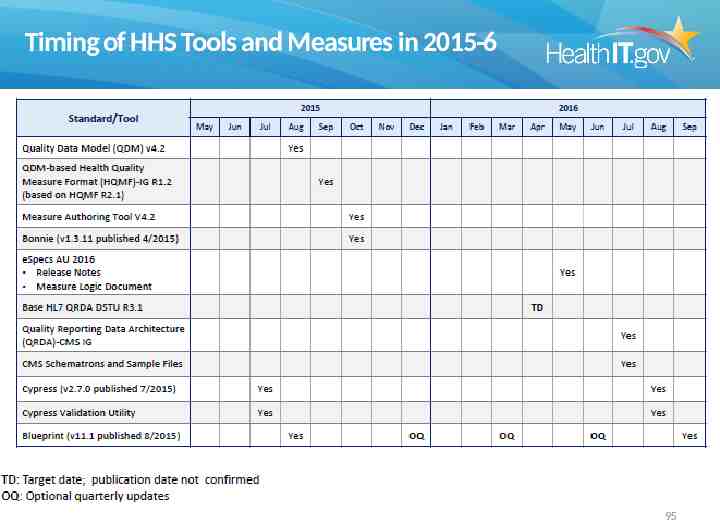
Timing of HHS Tools and Measures in 2015-6 95
Stay connected, communicate, and collaborate visit . . . Browse the ONC website at: healthIT.gov click the Facebook “Like” button to add us to your network Contact us at: [email protected] Visit the Health IT Dashboard: dashboard.healthit.gov Make speaker requests here: http://www.healthit.gov/requestspeaker Subscribe, watch, and share: @ONC HealthIT http://www.youtube.com/user/HHSONC Health IT and Electronic Health Records http://www.scribd.com/HealthIT/ http://www.flickr.com/photos/healthit Health IT Buzz Blog Visit the ONC Newsroom for news and announcements






