SDTMs in Medical Devices A First Attempt Phil Hall Distinguished
38 Slides2.58 MB

SDTMs in Medical Devices A First Attempt Phil Hall Distinguished Statistical Programmer Edwards Lifesciences Irvine, CA

Introduction CDISC now required for pharmaceutical trials No date set for medical device trials Medical device companies getting prepared

Agenda What are Medical Devices? Similarities with Pharmaceutical Studies Use of Device Specific Domains Differences of Medical Device Trials – Procedure Data – CEC Adjudicated Data – Day 0 Future Work Questions

What is a Medical Device? A man-made device which is placed on, in or near the body to cure, treat, diagnose or prevent a disease. Phil Hall 2017
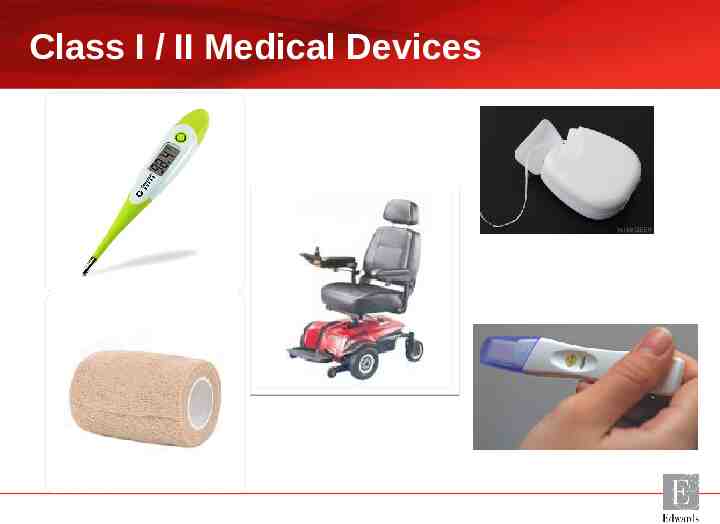
Class I / II Medical Devices
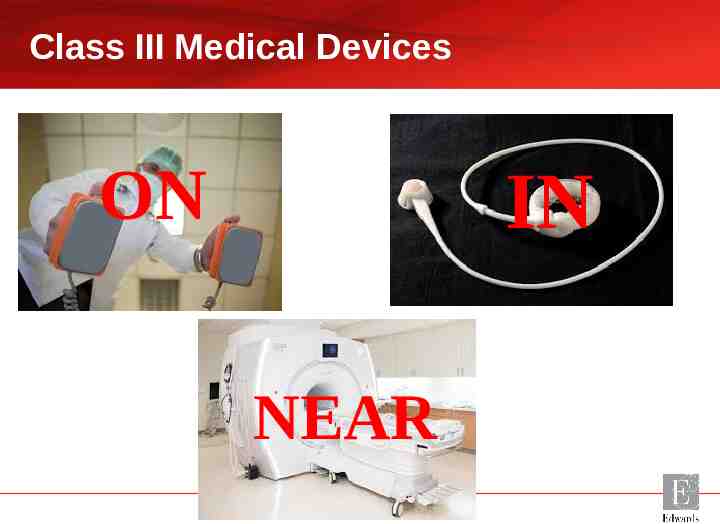
Class III Medical Devices ON IN NEAR
SAPIEN 3 Transcatheter Heart Valve http://www.edwards.com/therapies/transcatheter-aortic-valve-replacementtavr #WUSS16
Edwards Approach to SDTM Mapping CDISC Study Data Tabulation Model Implementation Guide (SDTM IG): Human Clinical Trials Version 3.2 Study Data Tabulation Model Implementation Guide for Medical Devices (SDTMIG-MD): Version 1.0 Keep as simple as possible Plan for the future – Used proposed domains for V3.3

Edwards Approach to SDTM Mapping 1) Low hanging fruit – AE, MH, LB, VS etc. – Using SUPPxx 2) Cross-form domains – DM, DS, SV 3) Others – SDTM Consistency Committee Propose – Debate - Agree
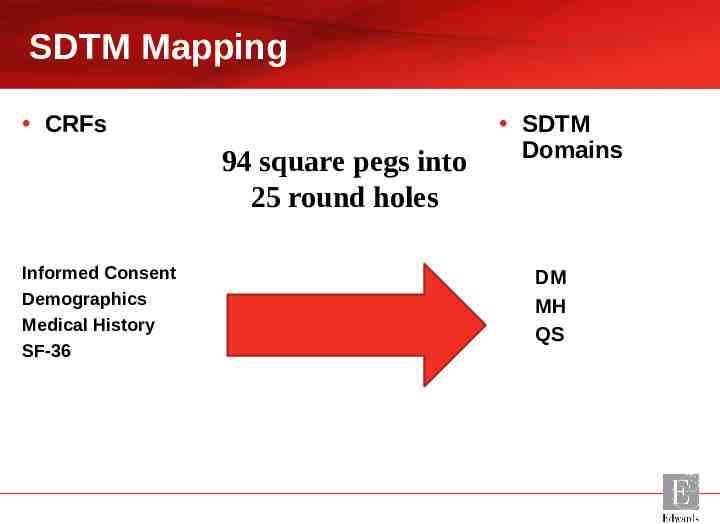
SDTM Mapping CRFs 94 square pegs into 25 round holes Informed Consent Demographics Medical History SF-36 SDTM Domains DM MH QS

Challenges SDTMs designed for drug trials not medical devices Multiple correct answers
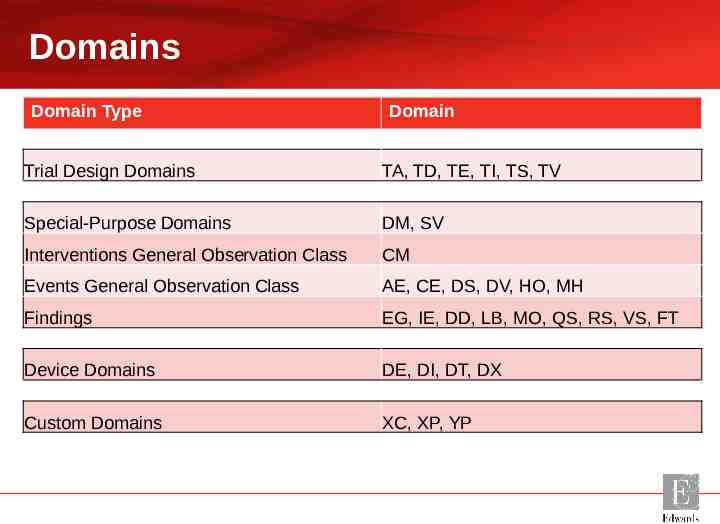
Domains Domain Type Domain Trial Design Domains TA, TD, TE, TI, TS, TV Special-Purpose Domains DM, SV Interventions General Observation Class CM Events General Observation Class AE, CE, DS, DV, HO, MH Findings EG, IE, DD, LB, MO, QS, RS, VS, FT Device Domains DE, DI, DT, DX Custom Domains XC, XP, YP

Trial Design Domains – – – – – – TA (Trial Arms) TD (Trial Disease Assessments) TE (Trial Elements) TI (Trial Inclusion / Exclusion Criteria) TS (Trial Summary Information) TV (Trial Visits)

Common Domains AE (Adverse Events) CM (Concomitant and Prior Medications) DD (Death Details) DM (Demographics) DS (Disposition) DV (Protocol Deviations) EG (ECG Results) FT (Functional Test – Proposed for V3.3)* IE (Inclusion / Exclusion Criteria Not Met) HO (Healthcare Encounters) LB (Laboratory Test Results) MH (Medical History) MO (Morphology) QS (Questionnaires) RS (Disease Response – currently only for oncology studies in V3.2 but being expanded for all indications in V3.3)** SV (Subject Visits) VS (Vital Signs) * What to Expect in SDTMIG v3.3, Fred Wood, PharmaSUG2015 http:// www.pharmasug.org/proceedings/2015/DS/PharmaSUG-2015-DS1 4.pdf ** https://www.cdisc.org/foundational/qrs
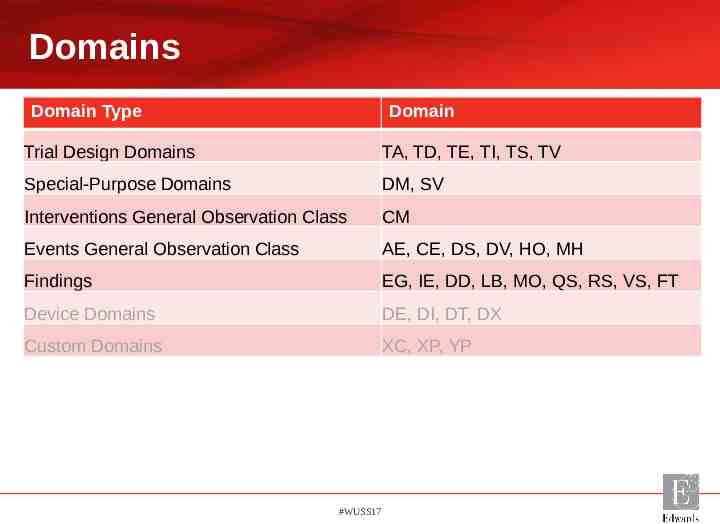
Domains Domain Type Domain Trial Design Domains TA, TD, TE, TI, TS, TV Special-Purpose Domains DM, SV Interventions General Observation Class CM Events General Observation Class AE, CE, DS, DV, HO, MH Findings EG, IE, DD, LB, MO, QS, RS, VS, FT Device Domains DE, DI, DT, DX Custom Domains XC, XP, YP #WUSS17
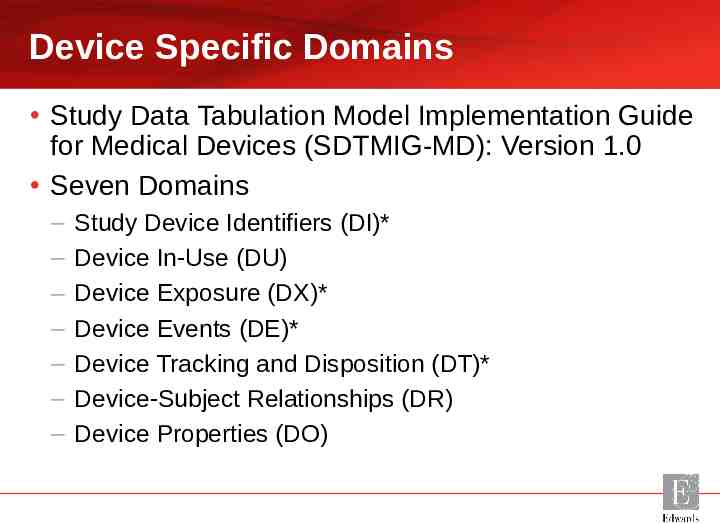
Device Specific Domains Study Data Tabulation Model Implementation Guide for Medical Devices (SDTMIG-MD): Version 1.0 Seven Domains – – – – – – – Study Device Identifiers (DI)* Device In-Use (DU) Device Exposure (DX)* Device Events (DE)* Device Tracking and Disposition (DT)* Device-Subject Relationships (DR) Device Properties (DO)
Study Device Identifiers (DI)* The primary purpose of this domain is to provide a consistent sponsor-defined variable (SPDEVID) for linking data across Device domains. Device Identifiers exist independently from subjects and therefore the Study DI domain does not contain USUBJID.

Device In-Use (DU) Device In-Use is a Findings domain that contains the values of measurements and settings that are intentionally set on a device when it is used, and may vary from subject to subject or other target. These are characteristics that exist for the device, and have a specific setting for a use instance.

Device Exposure (DX)* Device Exposure is an Interventions domain that records the details of a subject’s exposure to a medical device under study.

Device Events (DE)* DE is an Events domain that contains information about various kinds of device-related events, such as device malfunctions.

Device Tracking and Disposition (DT)* The Device Tracking and Disposition domain is an Events domain that represents a record of tracking events for a given device. This could include initial shipment, deployment, return, destruction, etc.

Device-Subject Relationships (DR) The Device-Subject Relationships domain is a special-purpose domain that links each subject to devices to which they may have been exposed.

Device Properties (DO) The Device Properties Findings domain is used to report characteristics of the device that are important to include in the submission, do not vary over the course of the study but are not used to identify the device. Examples include expiration date or shelf life.
Procedure Data Options – PR Domain – Device-specific Domain – Custom Domain

PR Domain PR – Assumptions for the Procedures Domain Model The Procedures domain model reflects collected details describing a subject’s therapeutic and diagnostic procedures. Some example procedures by type include the following: a. disease screening b. endoscopic examinations c. diagnostic tests d. therapeutic procedures e. surgical procedures (e.g., curative surgery, diagnostic surgery, palliative surgery, therapeutic surgery, prophylactic surgery, resection, stenting, hysterectomy, tubal ligation, implantation) CDISC Study Data Tabulation Model Implementation Guide (SDTM IG): Human Clinical Trials Version 3.2
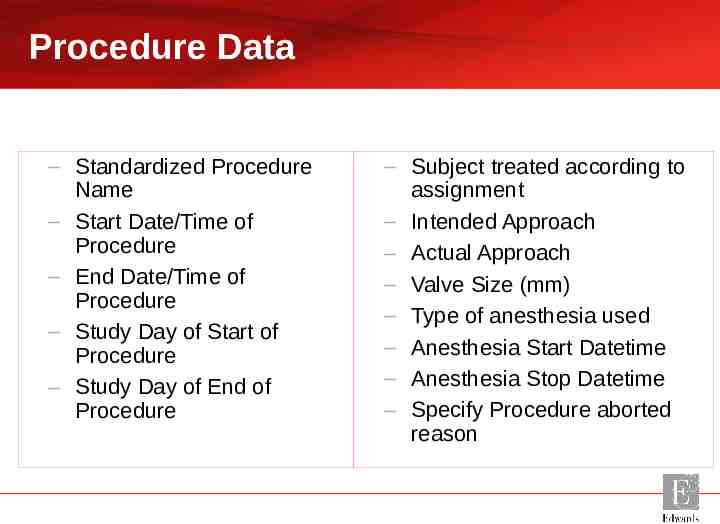
Procedure Data – Standardized Procedure Name – Start Date/Time of Procedure – End Date/Time of Procedure – Study Day of Start of Procedure – Study Day of End of Procedure – Subject treated according to assignment – Intended Approach – Actual Approach – Valve Size (mm) – Type of anesthesia used – Anesthesia Start Datetime – Anesthesia Stop Datetime – Specify Procedure aborted reason
Procedure Data Options – PR Domain – Device-specific Domain – Custom Domain X

Adjudicated Data Clinical Endpoint Committee (CEC) – Independent panel of experts reviewing clinical data to give expert opinion on certain events of interest. – More common in medical devices but not unique – Examples Disabling / Non-disabling Stroke Cardiovascular / Non-cardiovascular Mortality Re-hospitalization Due to Valve-related Symptoms
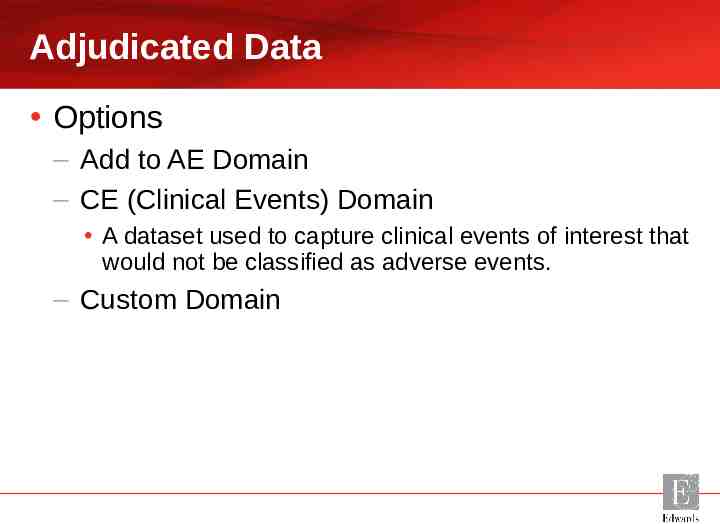
Adjudicated Data Options – Add to AE Domain – CE (Clinical Events) Domain A dataset used to capture clinical events of interest that would not be classified as adverse events. – Custom Domain
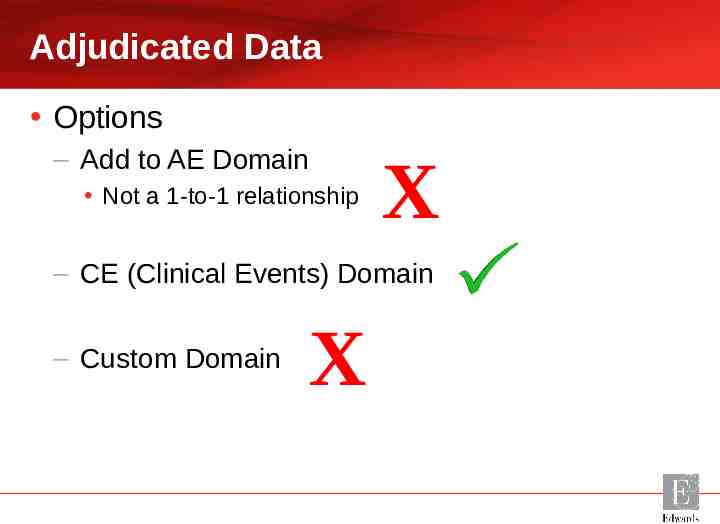
Adjudicated Data Options – Add to AE Domain Not a 1-to-1 relationship X – CE (Clinical Events) Domain – Custom Domain X

Day 0 vs Day 1 Medical Devices Day of Implant Procedure Day 0 Pharmaceutical Day of First Dose Day 1
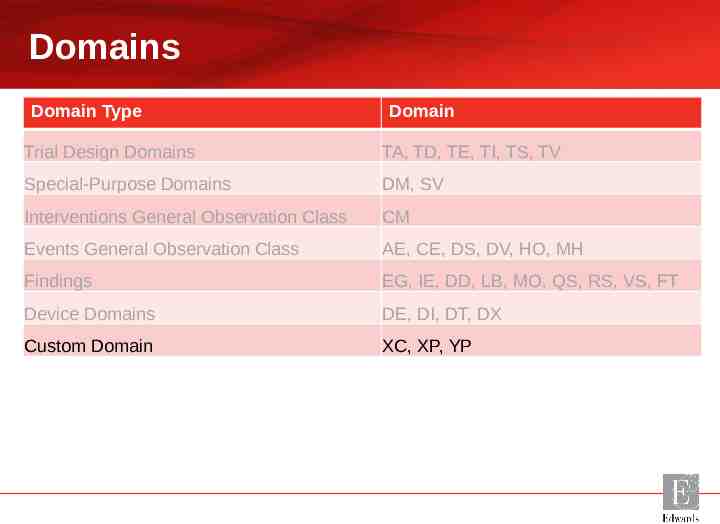
Domains Domain Type Domain Trial Design Domains TA, TD, TE, TI, TS, TV Special-Purpose Domains DM, SV Interventions General Observation Class CM Events General Observation Class AE, CE, DS, DV, HO, MH Findings EG, IE, DD, LB, MO, QS, RS, VS, FT Device Domains DE, DI, DT, DX Custom Domain XC, XP, YP
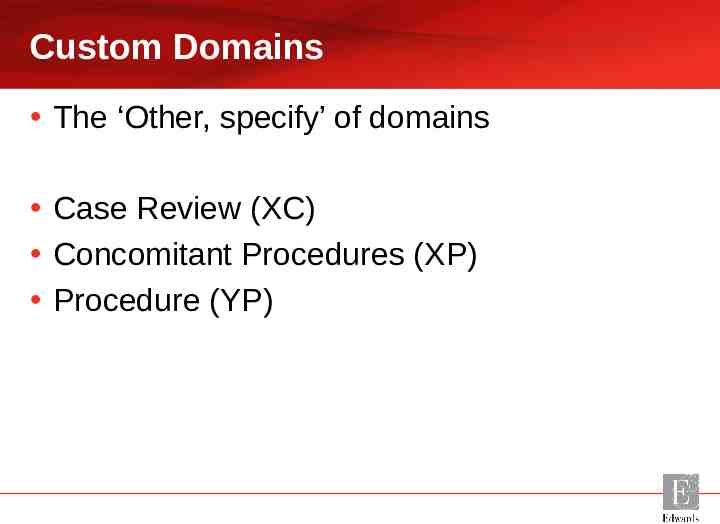
Custom Domains The ‘Other, specify’ of domains Case Review (XC) Concomitant Procedures (XP) Procedure (YP)
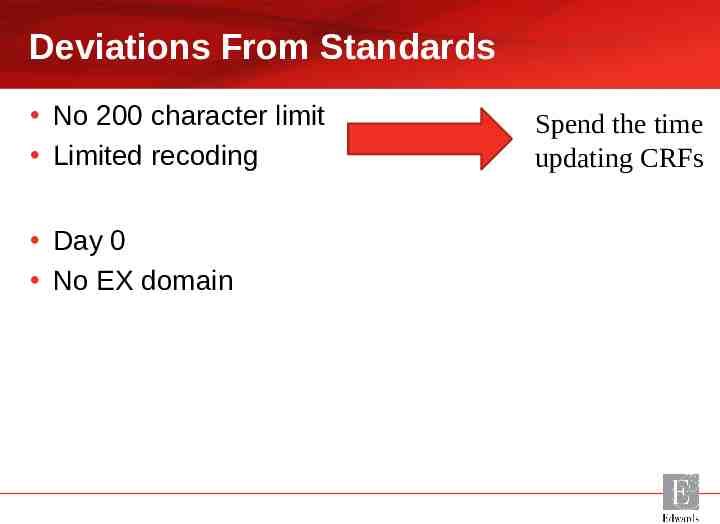
Deviations From Standards No 200 character limit Limited recoding Day 0 No EX domain Spend the time updating CRFs

Summary of Advice Use basic pharma domains where obvious to use – AE, MH, VS, LB etc. Create trial design domains Use device-specific domains where appropriate Use CE domain for adjudicated data Use custom domains for anything else Can’t be CDISC compliant without proper CRFs so keep first attempt simple
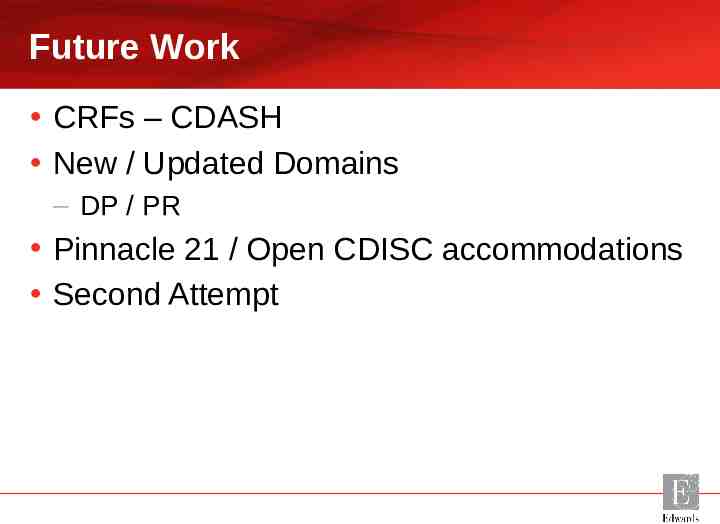
Future Work CRFs – CDASH New / Updated Domains – DP / PR Pinnacle 21 / Open CDISC accommodations Second Attempt

Questions? ?

Contact Details Phil Hall Edwards Lifesciences One Edwards Way Irvine CA 92614 949-250-4881 Phil [email protected] #WUSS17 38 #WUSS14






