Naming Covalent Compounds When it is all NONMETALS
35 Slides547.50 KB

Naming Covalent Compounds When it is all NONMETALS
Compounds vs Molecules A Compound is any substance composed of two or more DIFFERENT elements. A Molecule is any substance composed of two or more atoms COVALENTLY BONDED.

Properties of Covalent Compounds Generally Low Melting and Boiling Points Generally Soft and Flexible Tend to be Flammable Don’t conduct electricity Normally won’t dissolve in water
Types of Covalent Bonds Formed between two nonmetals in 14, 15, 16, and 17 Nonmetals have high electronegativity values Electrons are shared single bond shares one pair electrons double bond shares two pairs electrons triple bond shares three pairs electrons

Diatomic Elements Gases that exist as diatomic molecules are H2, F2, N2, O2, Cl2, Br2, I2 They are simply given their elements name. Exist this way only when not in compounds

Learning Check Use the name of the element to name the following diatomic molecules. H2 hydrogen N2 nitrogen Cl2 O2 I2

Naming Covalent Compounds Two nonmetals Name each element End the last element in -ide Add prefixes to show more than 1 atom Prefixes mon 1 hexa di 2 hepta tri 3 octa tetra 4 nona penta 5 deca 6 7 8 9 10
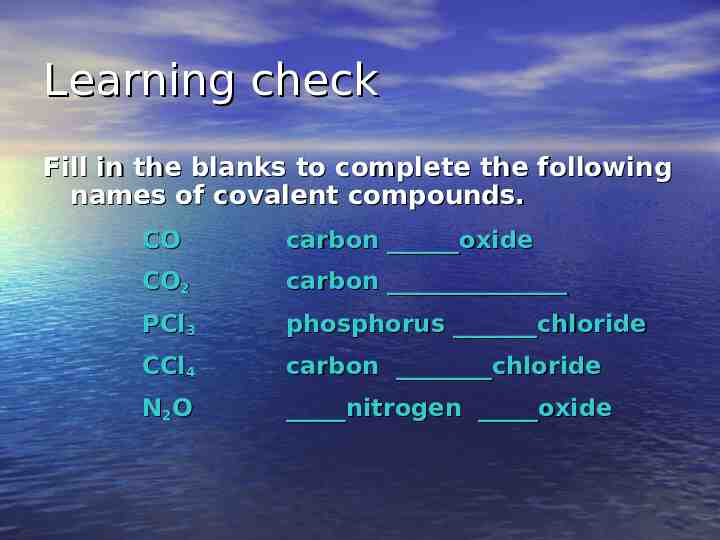
Learning check Fill in the blanks to complete the following names of covalent compounds. CO carbon oxide CO2 carbon PCl3 phosphorus chloride CCl4 carbon chloride N2O nitrogen oxide

Learning Check A. P2O5 1) phosphorus oxide 2) phosphorus pentoxide 3) diphosphorus pentoxide B. Cl2O7 1) dichlorine heptoxide 2) dichlorine oxide 3) chlorine heptoxide C. Cl2 1) chlorine 2) dichlorine 3) dichloride
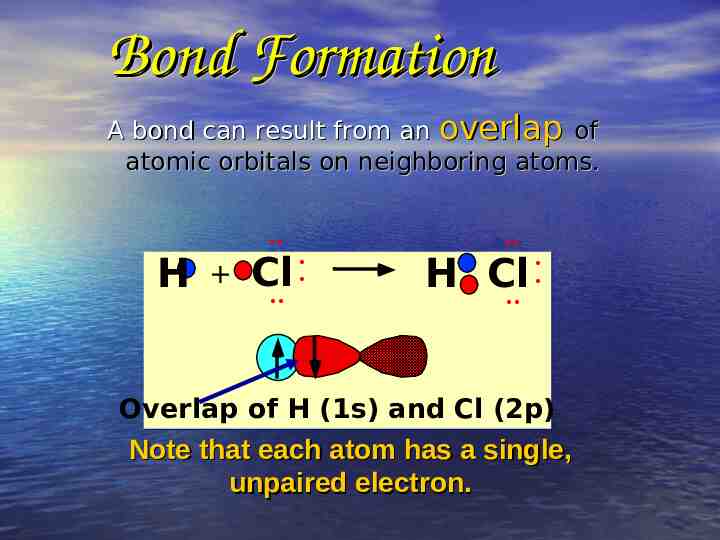
Bond Formation A bond can result from an overlap of atomic orbitals on neighboring atoms. H Cl H Cl Overlap of H (1s) and Cl (2p) Note that each atom has a single, unpaired electron.

Review Review of of Valence Valence Electrons Electrons Remember from the electron chapter that valence electrons are the electrons in the OUTERMOST energy level that’s why we did all those electron configurations! B is 1s2 2s2 2p1; so the outer energy level is 2, and there are 2 1 3 electrons in level 2. These are the valence electrons! Br is [Ar] 4s2 3d10 4p5 How many valence electrons are present?
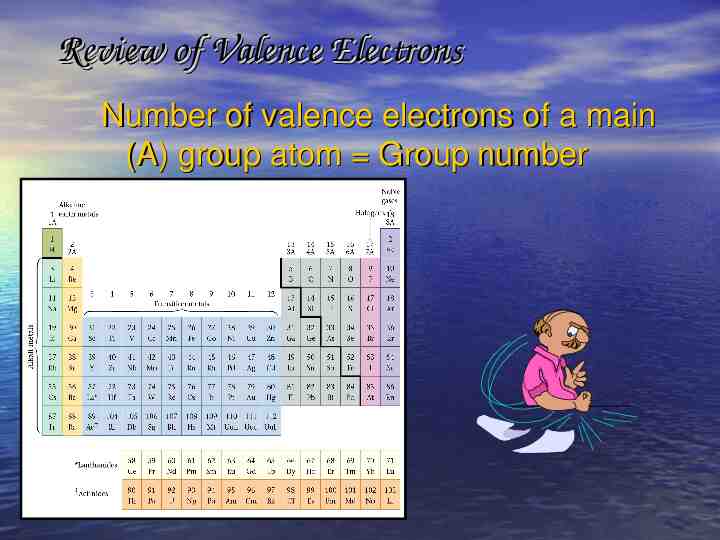
Review of Valence Electrons Number of valence electrons of a main (A) group atom Group number
Lewis Structures Drawings of covalent compounds Dots are used for nonbonding electrons Lines are used for bonding pairs of electrons All atoms must have 8 electrons in some combination except H which has 2

Lone pairs versus bonding pairs Lone pairs are pairs of electrons not in bonding Bonding pairs are pairs of electrons involved in bonding

Steps Building a Lewis Structure 1. Decide on the central atom; never H. Why? If there is a choice, the central atom is atom of lowest affinity for electrons. (Most of the time, this is the least electronegative atom the single atom is normally the lowest or the one further to the left on the Periodic Table.) 2. Add up the number of valence electrons that can be used.
Building a Dot Structure 3. Form a single bond between the central atom and each surrounding atom (each bond takes 2 electrons!) 4. Remaining electrons form LONE PAIRS to complete the octet as needed (or duet in the case of H).
Building a Dot Structure 5. Check to make sure there are 8 electrons around each atom except H. H should only have 2 electrons. This includes SHARED pairs. 6. Move nonbonding pairs if necessary to make double or triple bonds to insure the octet rule
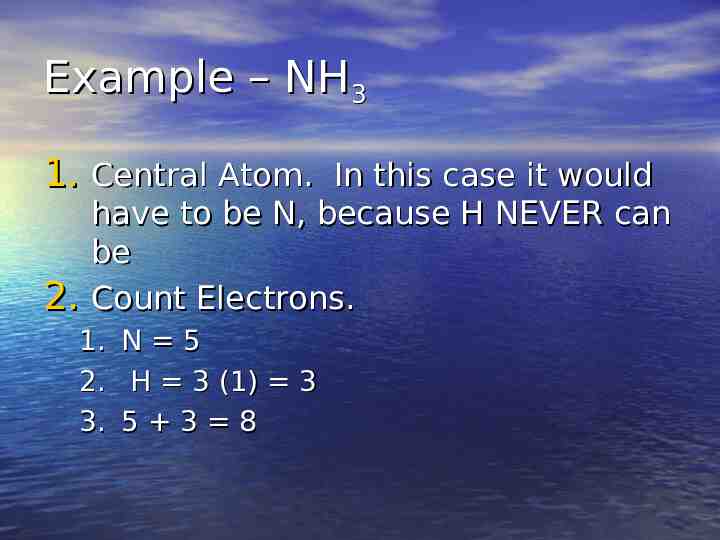
Example – NH3 1. Central Atom. In this case it would 2. have to be N, because H NEVER can be Count Electrons. 1. 2. 3. N 5 H 3 (1) 3 5 3 8
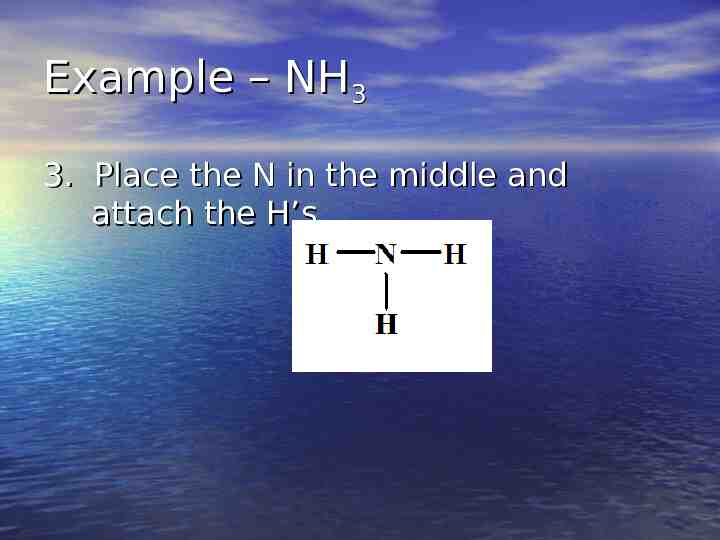
Example – NH3 3. Place the N in the middle and attach the H’s
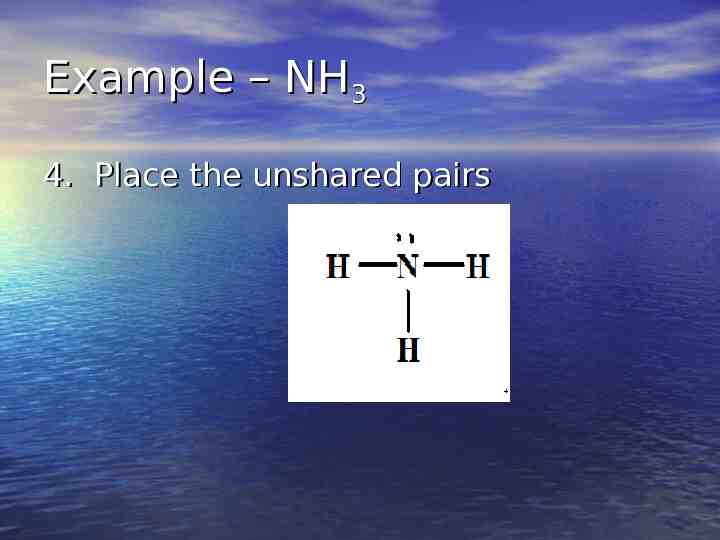
Example – NH3 4. Place the unshared pairs

Example – NH3 5. Count your electrons and check your work 1. 3 (2) 2 8 6. Add double or triple bonds if needed 1. None needed here so you are done
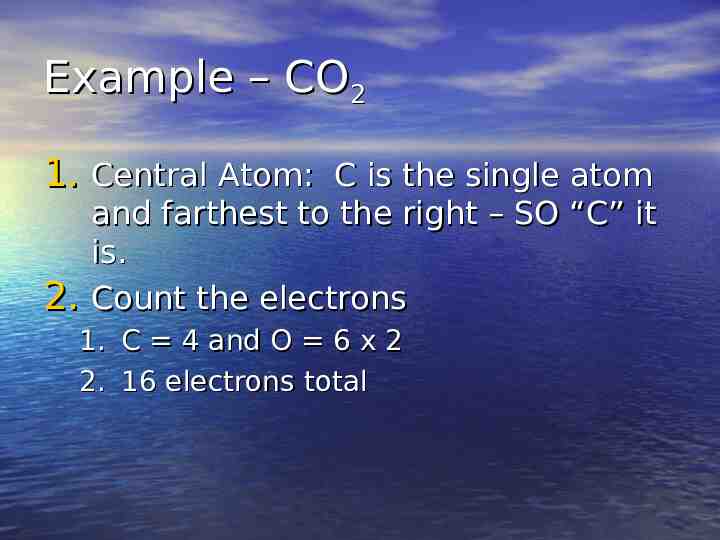
Example – CO2 1. Central Atom: C is the single atom 2. and farthest to the right – SO “C” it is. Count the electrons 1. C 4 and O 6 x 2 2. 16 electrons total
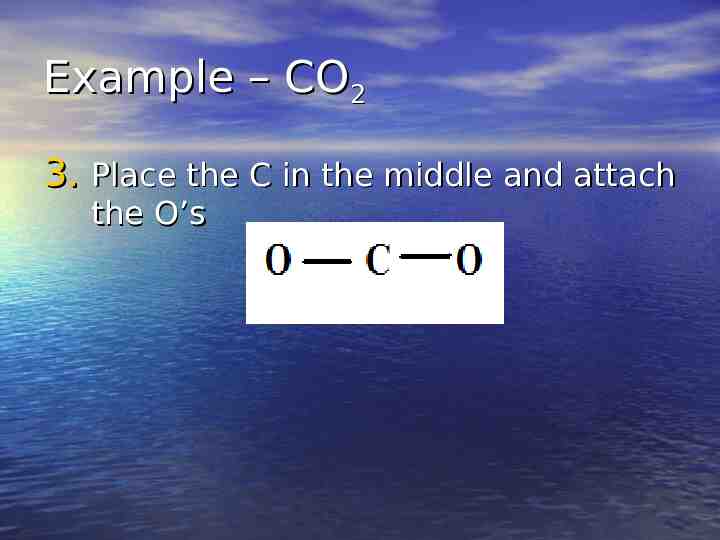
Example – CO2 3. Place the C in the middle and attach the O’s
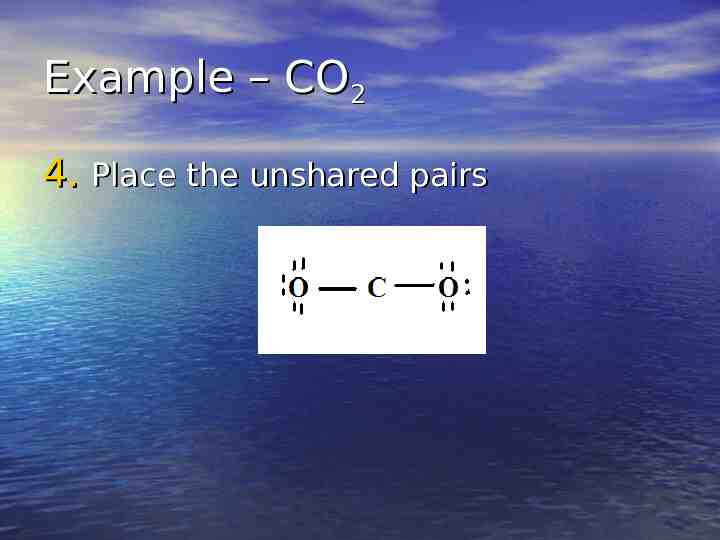
Example – CO2 4. Place the unshared pairs
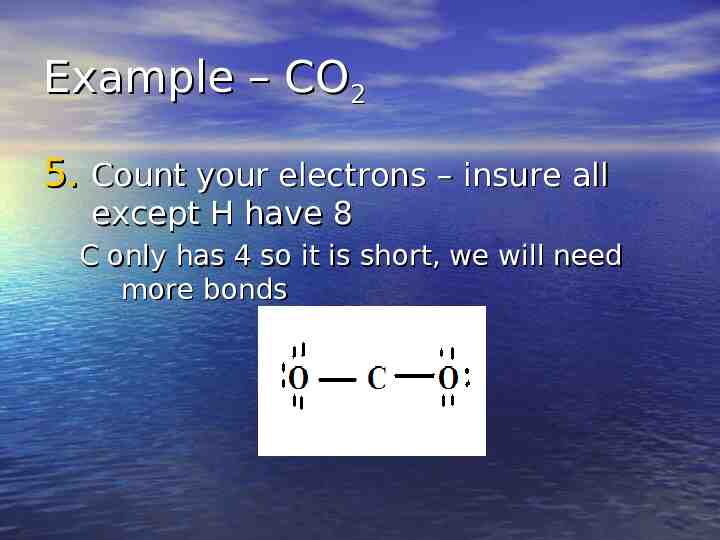
Example – CO2 5. Count your electrons – insure all except H have 8 C only has 4 so it is short, we will need more bonds
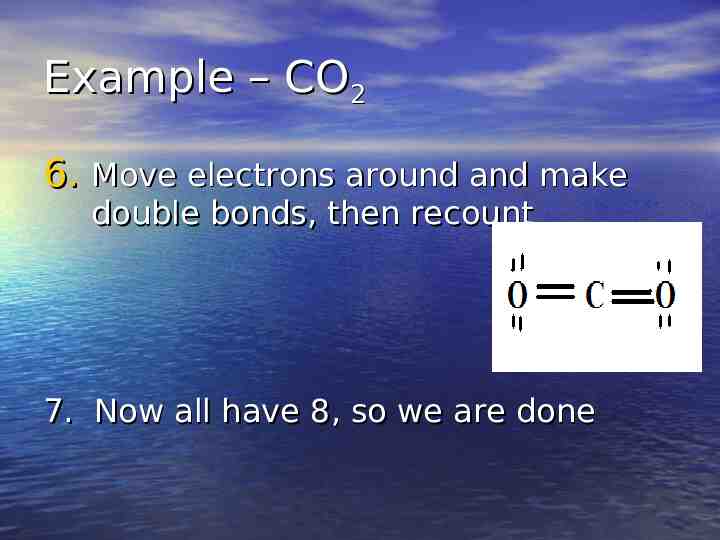
Example – CO2 6. Move electrons around and make double bonds, then recount 7. Now all have 8, so we are done

Example SO3-2 1. Central Atom here is “S” 2. Count electrons: 1. 2. 3. 4. S 6 O 6 x 3 18 -2 means add two more 6 18 2 26 total
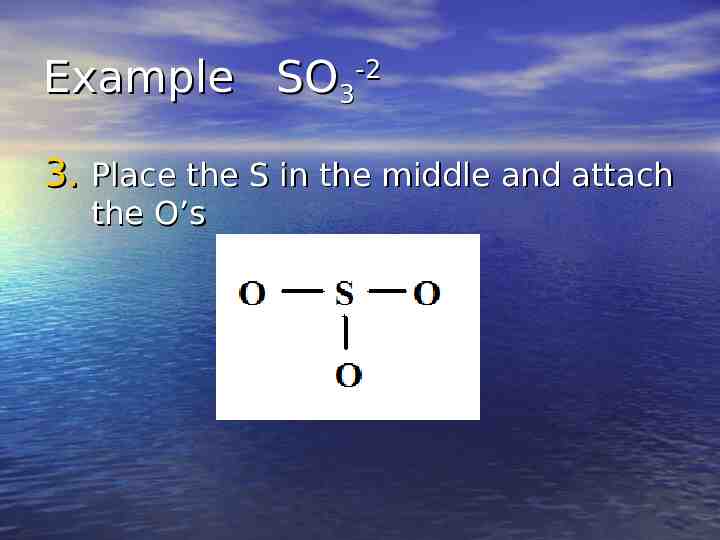
Example SO3-2 3. Place the S in the middle and attach the O’s

Example: SO3-2 4. Place the extra electrons 5. CHECK: All have 8 and I have placed 26 so I AM ALMOST DONE
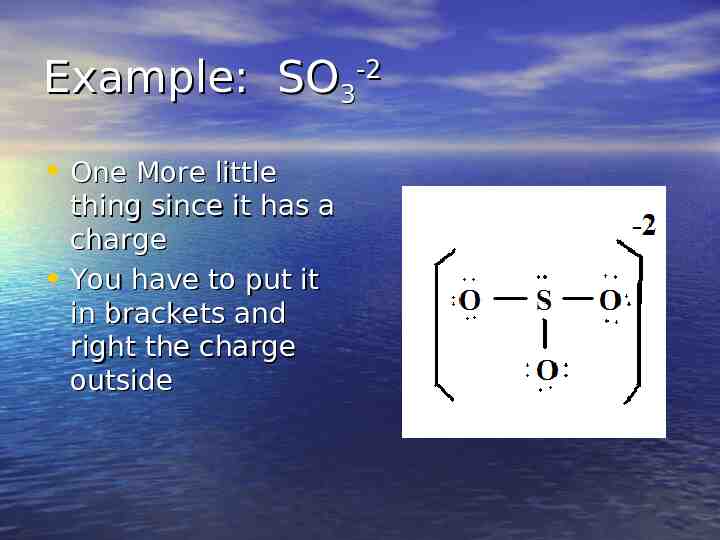
Example: SO3-2 One More little thing since it has a charge You have to put it in brackets and right the charge outside

VSEPR Valence Shell Electron Pair Repulsion Molecules take on shapes because electrons are all negative and attempt to repel as far away as possible. This Repulsion results in molecules taking on 3 dimensional Shapes

Shapes The Shapes are dependent on the areas of electron concentration – A single pair is an area of concentration – NON Bonding area – A single bond counts as an area of concentration – BONDING AREA – A double bond counts as an area of concentration – BONDING AREA – A triple bond count as an area of concentration – BONDING AREA
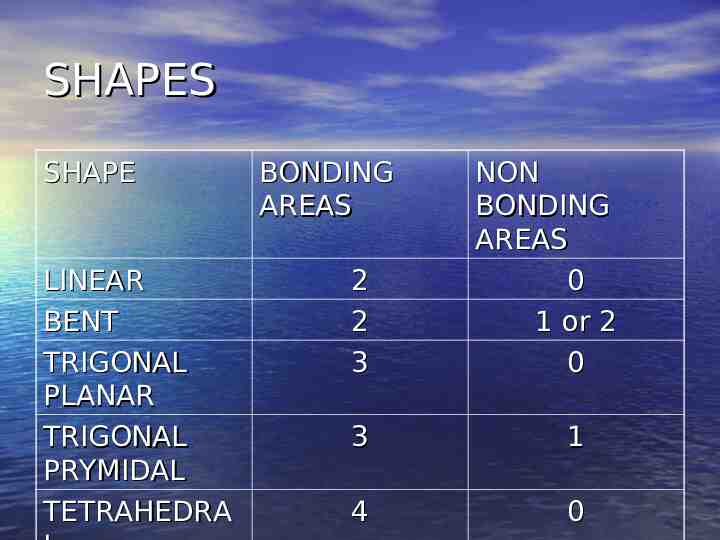
SHAPES SHAPE LINEAR BENT TRIGONAL PLANAR TRIGONAL PRYMIDAL TETRAHEDRA BONDING AREAS 2 2 3 NON BONDING AREAS 0 1 or 2 0 3 1 4 0
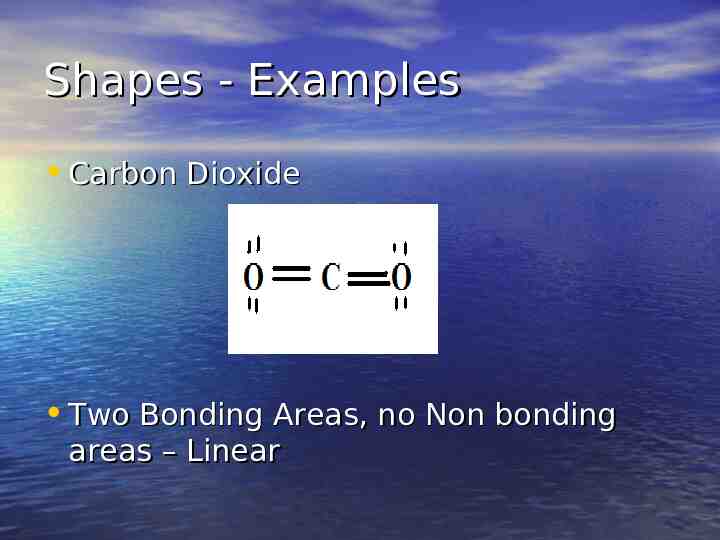
Shapes - Examples Carbon Dioxide Two Bonding Areas, no Non bonding areas – Linear
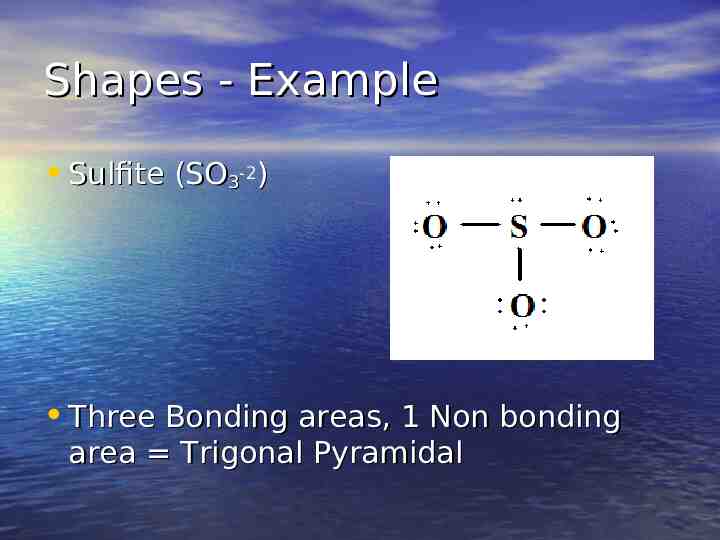
Shapes - Example Sulfite (SO3-2) Three Bonding areas, 1 Non bonding area Trigonal Pyramidal






