Engineered T cells: Next-generation cancer immunotherapy Rahul
24 Slides3.07 MB

Engineered T cells: Next-generation cancer immunotherapy Rahul Purwar, Assistant Professor Department of Biosciences & Bioengineering Indian Institute of Technology Bombay (IIT Bombay) Mumbai, India, 400076

Outline Introduction Adoptive T cell therapy: Engineered CAR-T cells Review of recent clinical trials Challenges of CAR T cell therapy Results T cell subsets: Th17 and Th9 cells in cancer immunotherapy Summary Adoptive T cell therapy: what we learnt and what we should do next
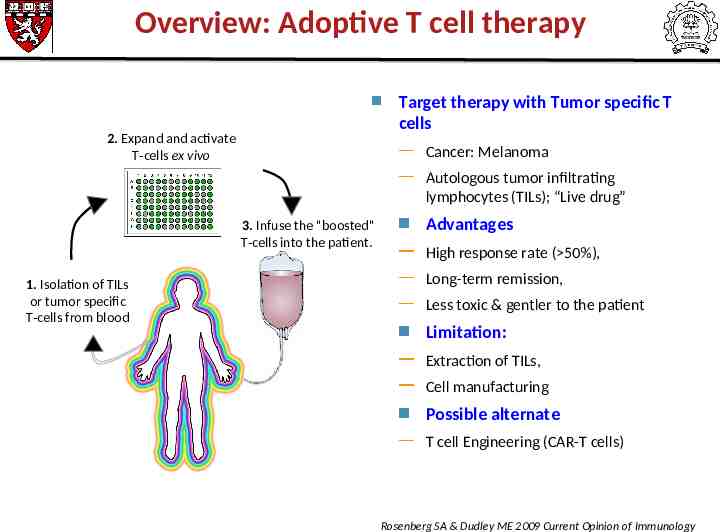
Overview: Adoptive T cell therapy Target therapy with Tumor specific T cells 2. Expand and activate T-cells ex vivo Cancer: Melanoma Autologous tumor infiltrating lymphocytes (TILs); “Live drug” 3. Infuse the "boosted" T-cells into the patient. 1. Isolation of TILs or tumor specific T-cells from blood Advantages High response rate ( 50%), Long-term remission, Less toxic & gentler to the patient Limitation: Extraction of TILs, Cell manufacturing Possible alternate T cell Engineering (CAR-T cells) Rosenberg SA & Dudley ME 2009 Current Opinion of Immunology
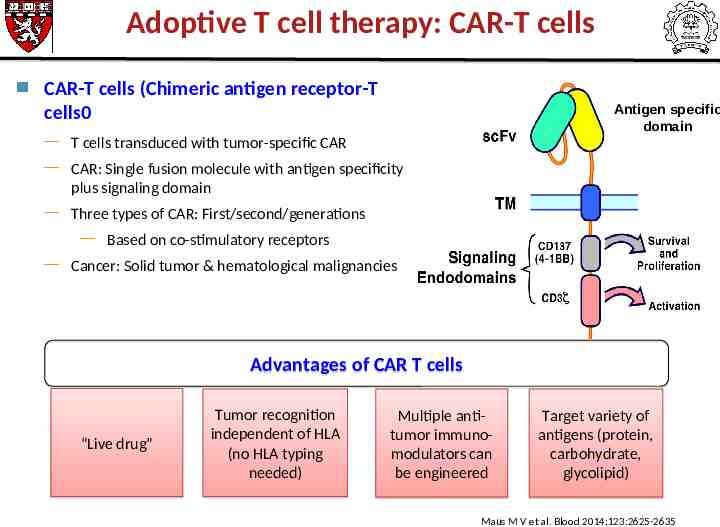
Adoptive T cell therapy: CAR-T cells CAR-T cells (Chimeric antigen receptor-T cells0 Antigen specific domain T cells transduced with tumor-specific CAR CAR: Single fusion molecule with antigen specificity plus signaling domain Three types of CAR: First/second/generations Based on co-stimulatory receptors Cancer: Solid tumor & hematological malignancies Advantages of CAR T cells “Live drug” Tumor recognition independent of HLA (no HLA typing needed) Multiple antitumor immunomodulators can be engineered Target variety of antigens (protein, carbohydrate, glycolipid) Maus M V et al. Blood 2014;123:2625-2635
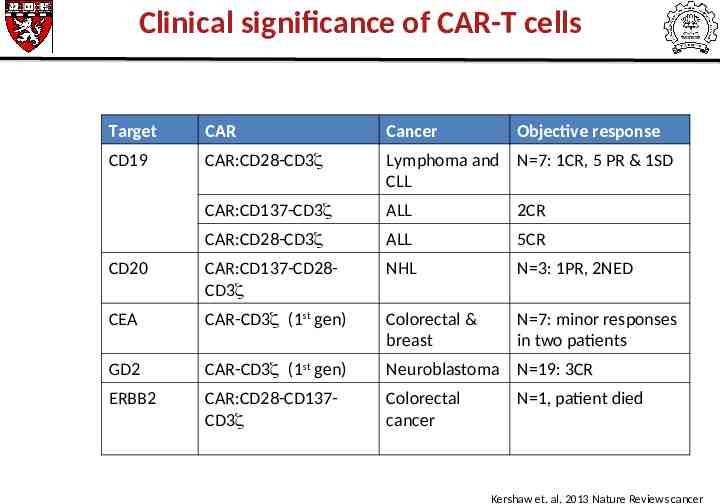
Clinical significance of CAR-T cells Target CAR Cancer Objective response CD19 CAR:CD28-CD3 Lymphoma and N 7: 1CR, 5 PR & 1SD CLL CAR:CD137-CD3 ALL 2CR CAR:CD28-CD3 ALL 5CR CD20 CAR:CD137-CD28CD3 NHL N 3: 1PR, 2NED CEA CAR-CD3 (1st gen) Colorectal & breast N 7: minor responses in two patients GD2 CAR-CD3 (1st gen) Neuroblastoma N 19: 3CR ERBB2 CAR:CD28-CD137CD3 Colorectal cancer N 1, patient died Kershaw et. al. 2013 Nature Reviews cancer
Challenges of CAR-T cells Toxicities On target/off tumor toxicities Metastatic colon cancer patient died after 5 days of infusion of ERBB2 CAR-T cells Low levels of ERBB2 express on lung epithelium (lung tox) Renal cell carcinoma: 5/11 patients developed liver toxicity Cytokine syndrome Elevated levels of pro-inflammatory cytokines Treatable by anti-IL-6mAb and steroids
Determinants of successful ACT: CAR-T cells Tumor target Efficacy & Long-term persistence Target antigen is critical determinant for efficacy & safety Subtypes of CD4 T cells (Th1, Th2, Th17, Th9 cells), Ideal target uniquely express on tumor cells or on cells which are not essential for survival CD8 T cells naïve, central memory; long-term effector; active but short lived Trafficking of CAR T cells to tumor Expression of addressins Route of CAR-T cell infusion Intra-tumoral/intravenous Optimal co-stimulation of T cells Patient conditioning before ACT Reduced-intensity or nonmyeloablative Increased intensity myelo ablative

Outline Introduction Adoptive T cell therapy: focus on engineered CAR-T cells Overview of current & investigational CAR therapies Challenges of CAR T cell therapy Results Roles of T-helper cell subsets in cancer immunotherapy Summary Adoptive T cell therapy: what we learnt and what we should do next
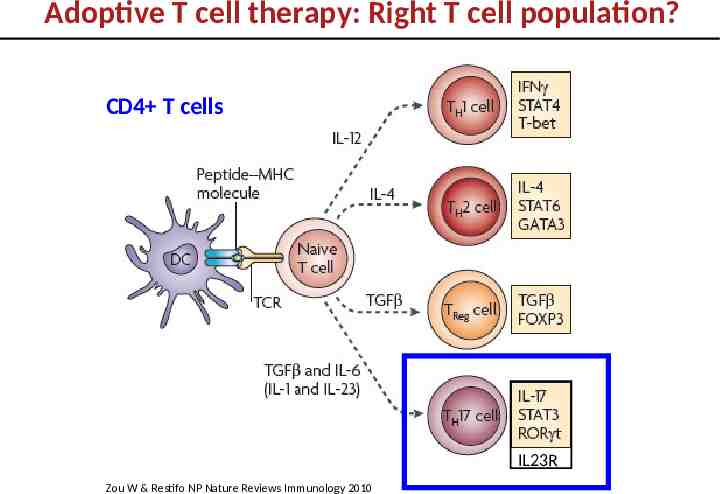
Adoptive T cell therapy: Right T cell population? CD4 T cells IL23R Zou W & Restifo NP Nature Reviews Immunology 2010
Role of Th17 cells in tumor immunity is controversial Pro-tumor: - enhances vascularization/angiogenesis - promotes metastasis - promotes growth Langowski et al, Nature 2006; Murugayen 2011 J.Immunol; Wang et al. J.Exp.Med 2009 Anti-tumor: - enhances tumor immunity by promoting CD8 T cell and DC function Martin-Orcazzo et al Immunity 2010
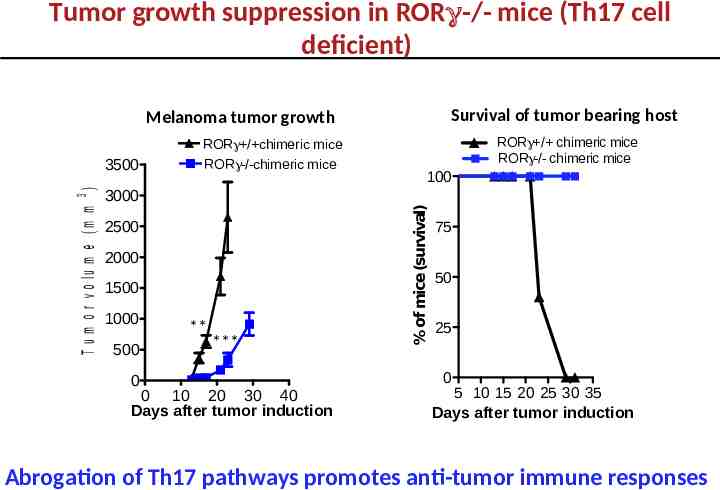
Tumor growth suppression in RORγ-/- mice (Th17 cell deficient) Survival of tumor bearing host Melanoma tumor growth 3000 2500 2000 1500 1000 500 0 ** *** 0 10 20 30 40 Days after tumor induction RORg / chimeric mice RORg-/- chimeric mice 100 % of mice (survival) T u m o r v o lu m e (m m 3 ) 3500 ROR / chimeric mice ROR -/-chimeric mice 75 50 25 0 5 10 15 20 25 30 35 Days after tumor induction Abrogation of Th17 pathways promotes anti-tumor immune responses
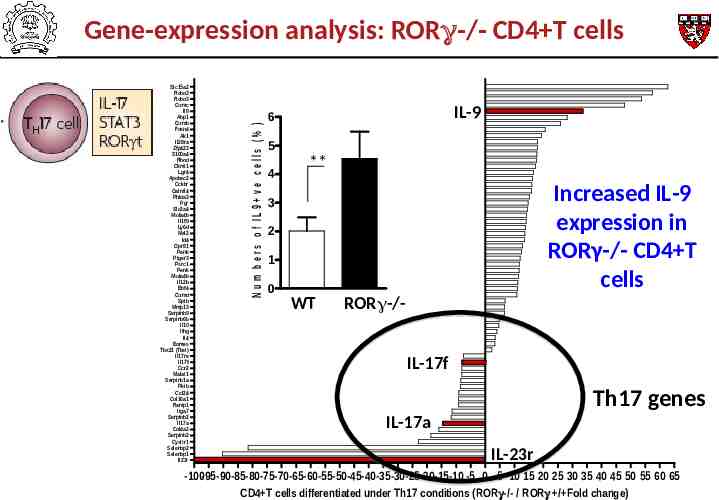
Slc15a2 Robo3 Robo3 Gzmc Il9 Abp1 Gzmb Foxn4 Ak1 Il28ra Zfp423 S100a4 Rhod Ckmt1 Lgr4 Apobec2 Cckbr Galntl4 Phlda3 Pgr Slc2a4 Ms4a4b Il1f9 Ly6d Mal2 Id4 Gpr81 Penk Ptger3 Psrc1 Penk Ms4a4b Il12b Ebf4 Gzmd Spib Mmp13 Serpinb9 Serpinb6b Il10 Ifng Il4 Eomes Tbx21 (Tbet) Il17re Il17f Ccr2 Malat1 Serpinb1a Pkib Ccl24 Col16a1 Ramp1 Itga7 Serpinb2 Il17a Col4a2 Serpinb2 Cysltr1 Selenbp2 Selenbp1 Il23r N u m b e r s o f IL 9 v e c e lls (% ) Gene-expression analysis: RORγ-/- CD4 T cells IL-9 6 5 4 ** Increased IL-9 expression in RORγ-/- CD4 T cells 3 2 1 0 WT ROR -/IL-17f Th17 genes IL-17a IL-23r -100-95-90-85-80-75-70-65-60-55-50-45-40-35-30-25-20-15-10 -5 0 5 10 15 20 25 30 35 40 45 50 55 60 65 CD4 T cells differentiated under Th17 conditions (ROR -/- / ROR / Fold change)
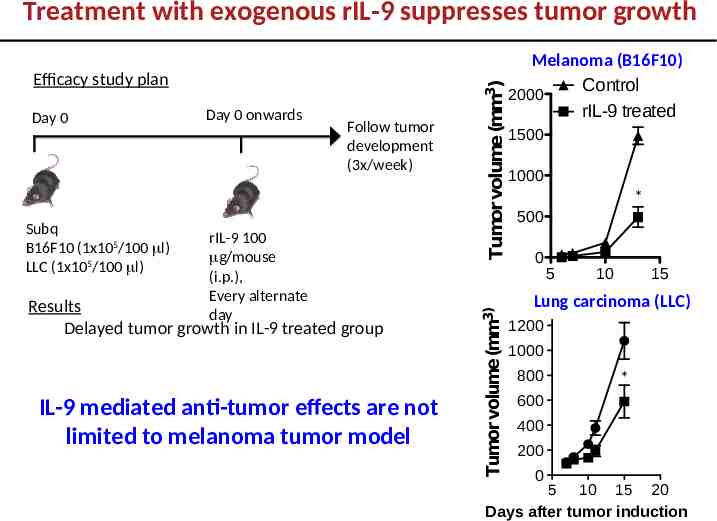
Day 0 Subq B16F10 (1x105/100 l) LLC (1x105/100 l) Day 0 onwards Follow tumor development (3x/week) rIL-9 100 g/mouse (i.p.), Every alternate day Results Delayed tumor growth in IL-9 treated group IL-9 mediated anti-tumor effects are not limited to melanoma tumor model Tumor volume (mm3) Efficacy study plan Tumor volume (mm3) Treatment with exogenous rIL-9 suppresses tumor growth Melanoma (B16F10) Control 2000 rIL-9 treated 1500 1000 * 500 0 5 10 15 control Lung carcinoma (LLC) rIL-9 treated 1200 1000 800 * 600 400 200 0 5 10 15 20 Days after tumor induction
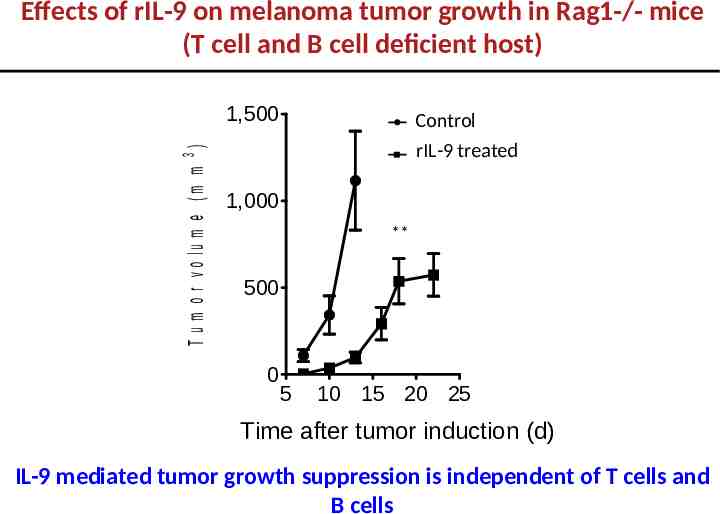
Effects of rIL-9 on melanoma tumor growth in Rag1-/- mice (T cell and B cell deficient host) T u m o r v o lu m e ( m m 3 ) 1,500 Control rIL-9 treated 1,000 ** 500 0 5 10 15 20 25 Time after tumor induction (d) IL-9 mediated tumor growth suppression is independent of T cells and B cells
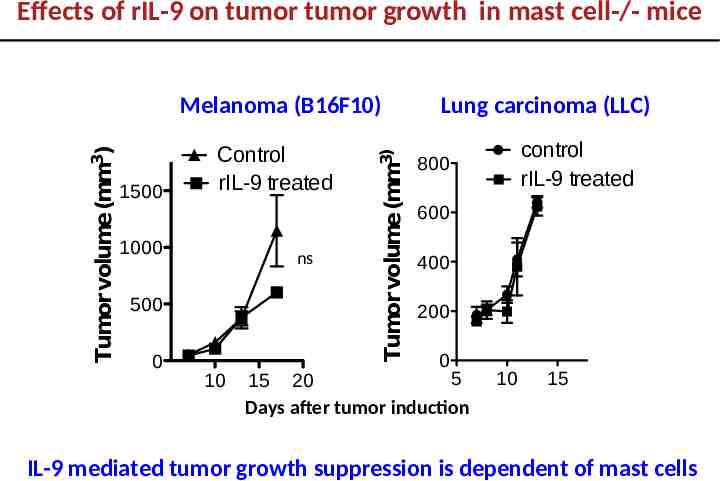
Effects of rIL-9 on tumor tumor growth in mast cell-/- mice 1500 Control rIL-9 treated 1000 ns 500 0 10 Tumor volume (mm3) Tumor volume (mm3) Melanoma (B16F10) Lung carcinoma (LLC) control rIL-9 treated 800 600 400 200 0 5 15 20 Days after tumor induction 10 15 IL-9 mediated tumor growth suppression is dependent of mast cells
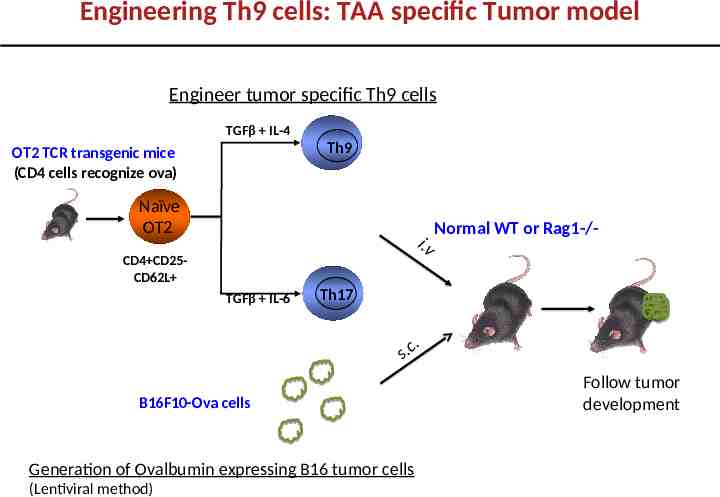
Engineering Th9 cells: TAA specific Tumor model Engineer tumor specific Th9 cells TGFβ IL-4 Th9 OT2 TCR transgenic mice (CD4 cells recognize ova) Naïve OT2 i.v CD4 CD25CD62L TGFβ IL-6 Normal WT or Rag1-/- Th17 .s c. B16F10-Ova cells Generation of Ovalbumin expressing B16 tumor cells (Lentiviral method) Follow tumor development
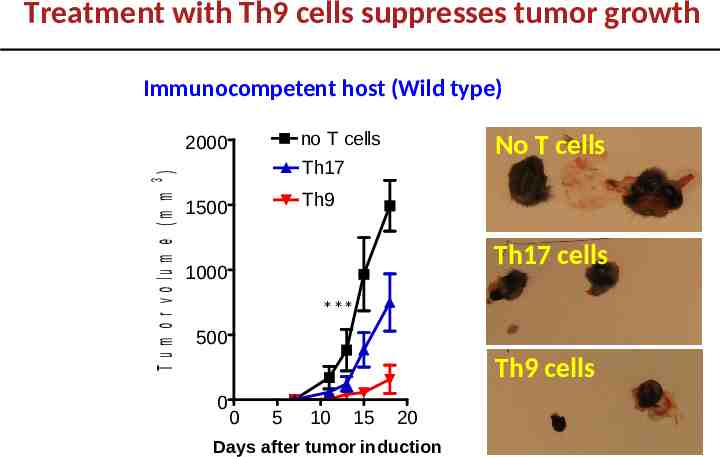
Treatment with Th9 cells suppresses tumor growth Immunocompetent host (Wild type) no T cells T u m o r v o lu m e (m m 3 ) 2000 No T cells Th17 Th9 1500 Th17 cells 1000 *** 500 Th9 cells 0 0 5 10 15 20 Days after tumor induction
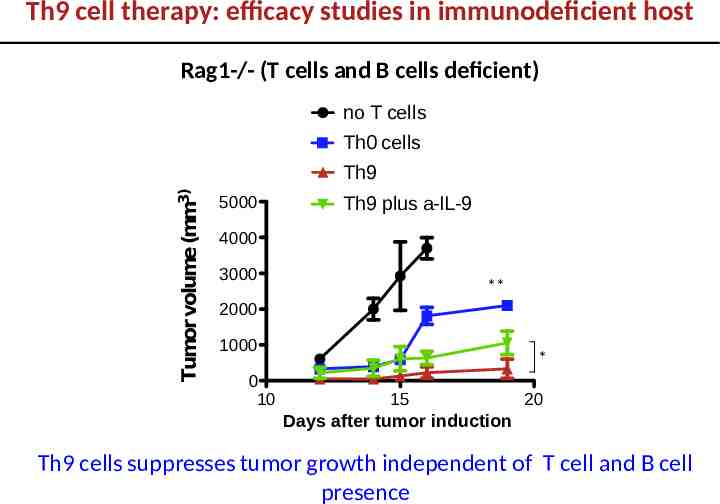
Th9 cell therapy: efficacy studies in immunodeficient host Rag1-/- (T cells and B cells deficient) no T cells Tumor volume (mm3) Th0 cells Th9 5000 Th9 plus a-IL-9 4000 3000 ** 2000 1000 0 10 * 15 20 Days after tumor induction Th9 cells suppresses tumor growth independent of T cell and B cell presence
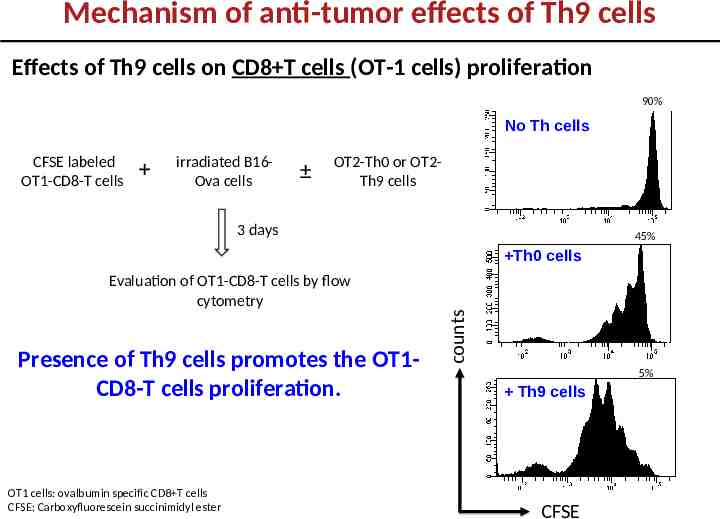
Mechanism of anti-tumor effects of Th9 cells Effects of Th9 cells on CD8 T cells (OT-1 cells) proliferation 90% No Th cells CFSE labeled OT1-CD8-T cells irradiated B16Ova cells OT2-Th0 or OT2Th9 cells 3 days 45% Evaluation of OT1-CD8-T cells by flow cytometry Presence of Th9 cells promotes the OT1CD8-T cells proliferation. OT1 cells: ovalbumin specific CD8 T cells CFSE: Carboxyfluorescein succinimidyl ester counts Th0 cells 5% Th9 cells CFSE
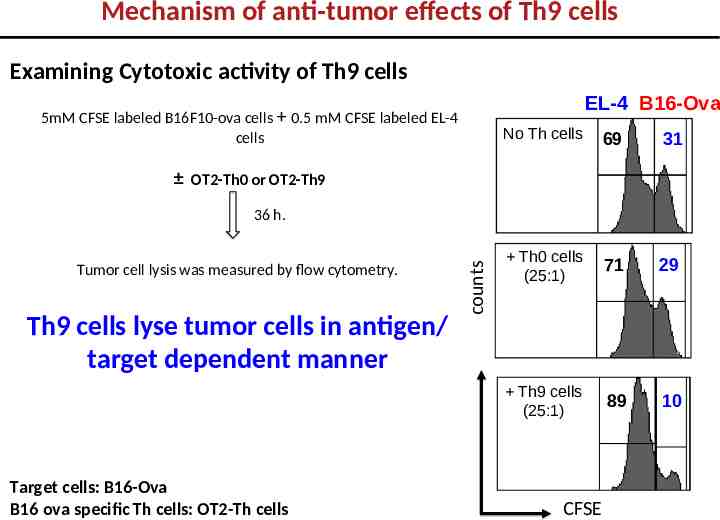
Mechanism of anti-tumor effects of Th9 cells Examining Cytotoxic activity of Th9 cells EL-4 B16-Ova 5mM CFSE labeled B16F10-ova cells 0.5 mM CFSE labeled EL-4 cells No Th cells 69 31 Th0 cells (25:1) 71 29 Th9 cells (25:1) 89 10 OT2-Th0 or OT2-Th9 Tumor cell lysis was measured by flow cytometry. Th9 cells lyse tumor cells in antigen/ target dependent manner Target cells: B16-Ova B16 ova specific Th cells: OT2-Th cells counts 36 h. CFSE
What is the relevance of these findings in human? Translational research
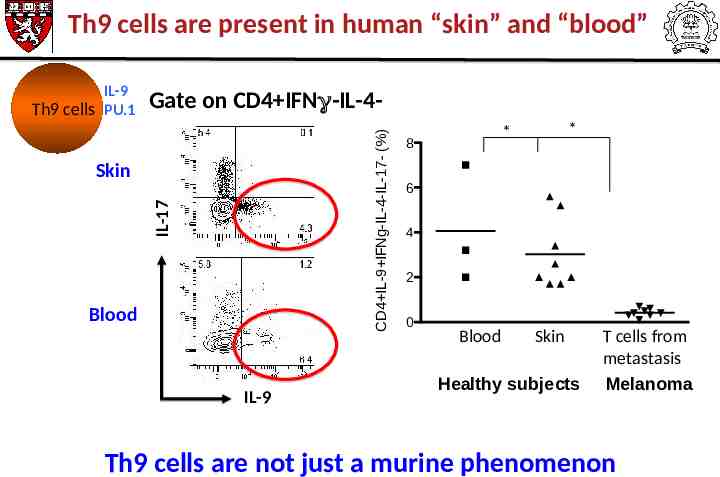
Th9 cells are present in human “skin” and “blood” Gate on CD4 IFN -IL-4CD4 IL-9 IFNg-IL-4-IL-17- (%) IL-9 Th9 cells PU.1 IL-17 Skin Blood IL-9 * * 8 6 4 2 0 Blood Skin Healthy subjects T cells from metastasis Melanoma Th9 cells are not just a murine phenomenon
Summary CAR-T cells Limitation of CAR-T cells T cells transduced with tumor-specific Chimeric Antigen Receptor (CAR) Tumor recognition independent of HLA (no HLA typing needed) Target: variety of tumor antigens (protein, carbohydrate, glycolipid) High response rate (up to 88%): pre-clinical and clinical findings Toxicities On target/off tumor toxicities Cytokine syndrome Tumor microenvironment Presence of MDSCs & Treg in tumor Immunosuppressive agents Results & Conclusion IL-9 is a novel anti-tumor cytokine and anti-tumor effects are mediated via mast cells Th9 cells are the most superior anti-tumor Th cells Th9 cells exists in human: not just murine phenomenon Strategies that promotes IL-9 production will be a critical for the development of robust treatment for melanoma and lung carcinoma.

Acknowledgement Laboratory members at IIT Bombay Richa Agarwal Mukti Vats Farha Memon Sathya Atish Department of Dermatology, Brigham & Women’s Hospital (BWH) Thomas S Kupper Department of Neurology, BWH Vijay K Kuchroo Wassim Elymann Sheng Xiao National Institute of Health (NIH/NIEHS) Anton J Jetten Hong Soon Kang !!Thanks a lot!!