Controlled Substances Rules & Regulations C AT H E R I N E P H A M , P
36 Slides1.28 MB

Controlled Substances Rules & Regulations C AT H E R I N E P H A M , P H A R M D , R P H T E X A S C H I L D R E N ’ S H O S P I TA L S P E C I A LT Y P H A R M A C Y APRIL 5, 2018

OBJECTIVES Describe rules and laws that regulate the prescribing and dispensing of controlled substances in the state of Texas Define the corresponding responsibility of prescribers and dispensers in relation to controlled substances Recognize requirements of controlled substance prescriptions, including emergency authorization procedures Identify prescriber practices to prevent diversion and abuse of prescription medications

GOVERNING BODIES U.S. Department of Justice United States Code Controlled Substances Act Drug Enforcement Administration Enforces U.S. controlled substances laws and regulations Registration with DEA required to manufacture, distribute, prescribe, possess, analyze, or dispense controlled substances Texas State Board of Pharmacy Texas Controlled Substances Act Texas Controlled Substances Rules When federal law or regulations differ from state law or regulations, practitioners and pharmacies are required to abide by the more stringent aspects of both the federal and state requirements
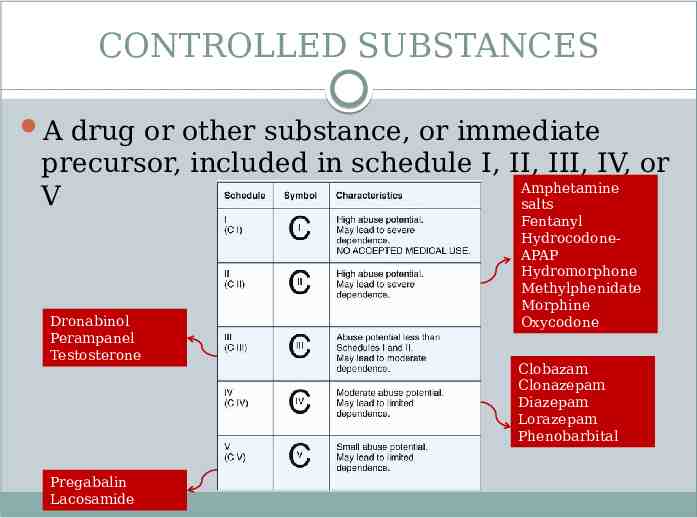
CONTROLLED SUBSTANCES A drug or other substance, or immediate precursor, included in schedule I, II, III, IV, or Amphetamine V salts Dronabinol Perampanel Testosterone Pregabalin Lacosamide Fentanyl HydrocodoneAPAP Hydromorphone Methylphenidate Morphine Oxycodone Clobazam Clonazepam Diazepam Lorazepam Phenobarbital
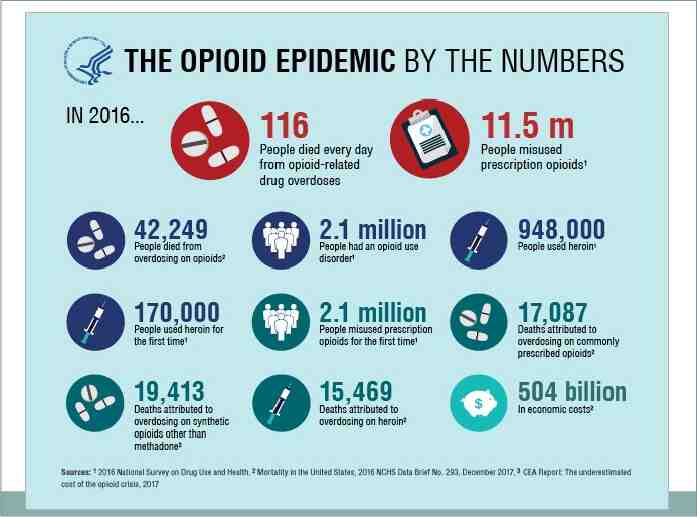
The majority of drug overdose deaths (66%) involve an opioid. Opioid overdoses accounted for more than 42,000 deaths in 2016, more than any previous year on record. Of these opioid overdose deaths, approximately what percentage involved a prescription opioid? a. b. c. d. 20% 40% 60% 80%
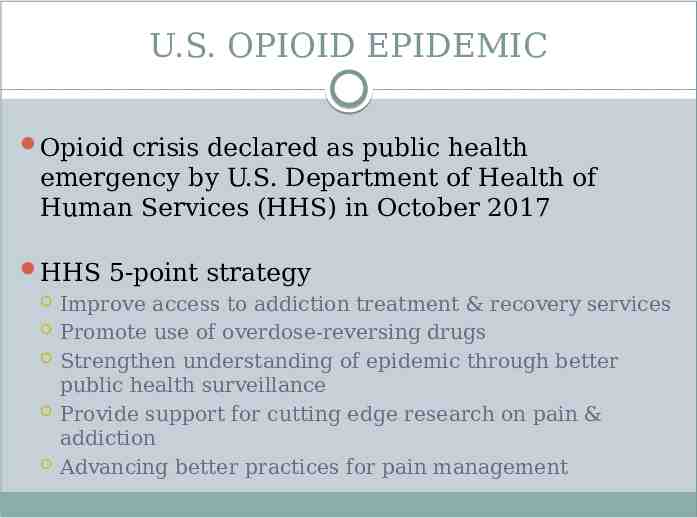
U.S. OPIOID EPIDEMIC Opioid crisis declared as public health emergency by U.S. Department of Health of Human Services (HHS) in October 2017 HHS 5-point strategy Improve access to addiction treatment & recovery services Promote use of overdose-reversing drugs Strengthen understanding of epidemic through better public health surveillance Provide support for cutting edge research on pain & addiction Advancing better practices for pain management
PRESCRIPTION DRUG ABUSE Misuse of prescription drugs Use without prescription Use in greater amounts, more often, or longer than directed Use in any other way not directed to be used Sources Friend/relative (given, stolen, purchased) Valid prescription Drug dealer Reasons for abuse Relieve physical pain Feel good, get high Relax, relieve tension Help with sleep

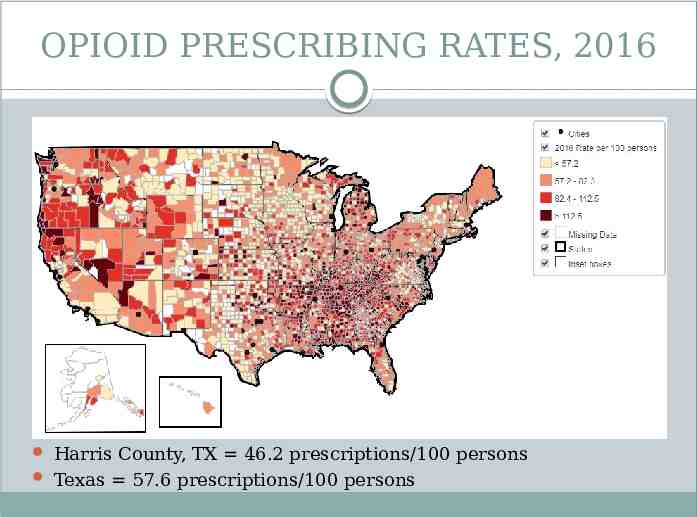
OPIOID PRESCRIBING RATES, 2016 Harris County, TX 46.2 prescriptions/100 persons Texas 57.6 prescriptions/100 persons
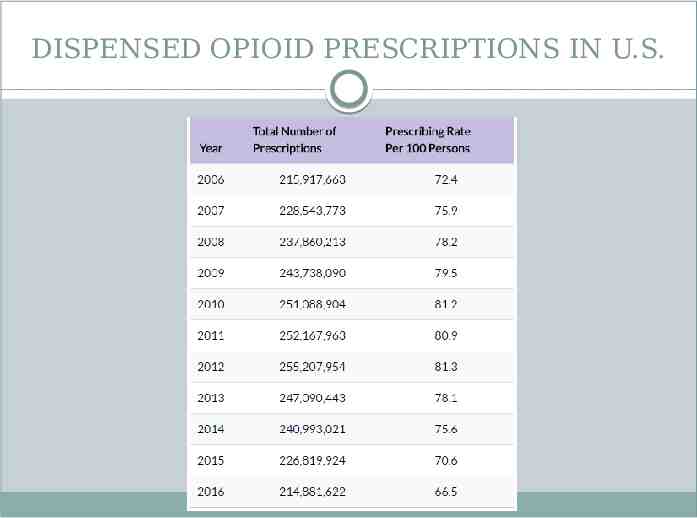
DISPENSED OPIOID PRESCRIPTIONS IN U.S.
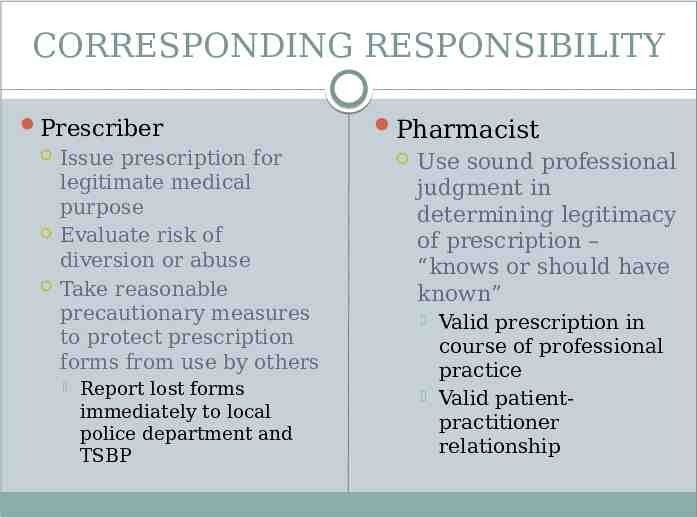
CORRESPONDING RESPONSIBILITY Controlled substances must only be prescribed, dispensed, delivered, or administered for valid medical purposes and in the course of medical practice “The responsibility for the proper prescribing and dispensing of controlled substances is upon the prescribing practitioner, but a corresponding responsibility rests with the pharmacist who fills the prescription”

CORRESPONDING RESPONSIBILITY Prescriber Issue prescription for legitimate medical purpose Evaluate risk of diversion or abuse Take reasonable precautionary measures to protect prescription forms from use by others Report lost forms immediately to local police department and TSBP Pharmacist Use sound professional judgment in determining legitimacy of prescription – “knows or should have known” Valid prescription in course of professional practice Valid patientpractitioner relationship
PRESCRIBERS Practitioner must be registered with DEA using DEA Form 224 and renewed every 3 years using DEA Form 224a Practitioner must maintain DEA Certificate of Registration, DEA Form 223, at registered location in readily retrievable manner Not required to register for other practice locations, unless administering or dispensing controlled substances Prescriptions may not be issued for purposes of general dispensing to patients

DESIGNATED AGENTS Communicate and prepare prescriptions on behalf of practitioner Practitioner personally responsible for actions of agent and cannot delegate: Medical determination of need Elements of valid prescription Signature authority Written designation must be available in practitioner’s usual place of business for inspection and pharmacist request

DELEGATION TO APRN & PA Prescriptive authority agreement – entered into by a physician and an advanced practice registered nurse (APRN) or physician assistant (PA) through which act of prescribing or ordering a drug/device is delegated Schedule II Hospital facility-based practices for patients admitted to hospital for intended length of stay 24 hours or patients receiving services in emergency department Hospice care patients with written certification of terminal illness Schedule III-V Prescription, including refill, cannot exceed 90 days Consultation with delegating physician and consultation noted in patient’s chart required for refill and for any child less than 2 years old

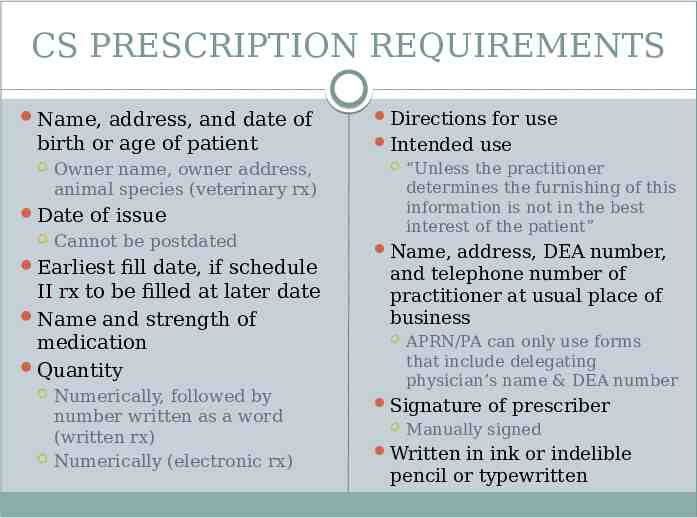
CS PRESCRIPTION REQUIREMENTS Name, address, and date of birth or age of patient Owner name, owner address, animal species (veterinary rx) Date of issue Cannot be postdated Earliest fill date, if schedule II rx to be filled at later date Name and strength of medication Quantity Numerically, followed by number written as a word (written rx) Numerically (electronic rx) Directions for use Intended use “Unless the practitioner determines the furnishing of this information is not in the best interest of the patient” Name, address, DEA number, and telephone number of practitioner at usual place of business APRN/PA can only use forms that include delegating physician’s name & DEA number Signature of prescriber Manually signed Written in ink or indelible pencil or typewritten
OFFICIAL PRESCRIPTION FORM
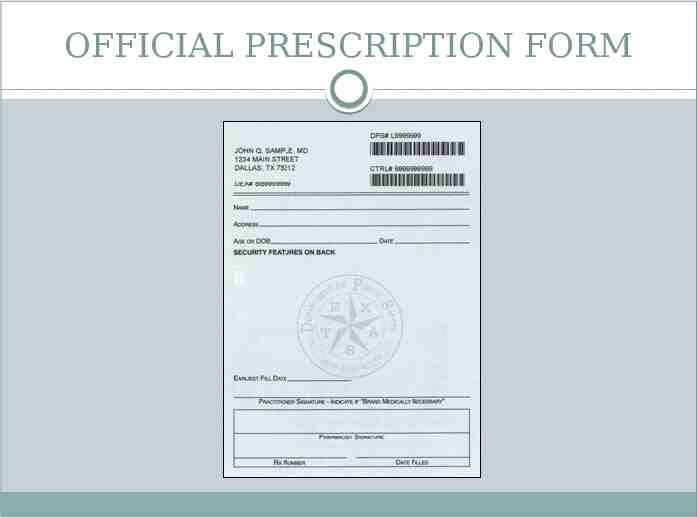
OFFICIAL PRESCRIPTION FORM Used to prescribe schedule II controlled substances Can only be ordered from Texas State Board of Pharmacy if practitioner is registered by DEA to prescribe schedule II controlled substances Must be sequentially numbered Only 1 prescription recorded on each form Legibly filled out by prescriber or designated agent Signed by dispensing pharmacist Dispense date Pharmacy prescription number Name, strength, quantity, dosage form of dispensed medication Retained by pharmacist for at least 2 years

CS PRESCRIPTIONS Schedule III-V Schedule II Can be prescribed via written, Must be prescribed via electronic, facsimile, oral, or official written telephonically communicated prescription form or prescription electronic prescription Cannot be refilled more than 5 Cannot be refilled times Cannot be filled or refilled Multiple prescriptions can later than 6 months after be issued at once for up to prescription issue date 90-day supply if “earliest Can be transferred between fill date” is indicated DEA-registered pharmacies for Must be dispensed within the purpose of refill dispensing 21 days after date of on a one-time basis only, unless pharmacies issuance or earliest fill electronically share real-time date online * Does not apply to medication orders written fordatabase patients admitted to a hospital

HOSPITAL DISCHARGE Patient can only possess controlled substance upon hospital release if medication is: Dispensed under medication order while admitted Placed in properly labeled container No more than 7-day supply

Lucille, an advanced practice registered nurse, has a prescriptive authority agreement with Dr. Adam Jones, MD. Assuming valid patient-practitioner relationship and legitimate medical need have been established, she is legally authorized to a. Call in a prescription to the pharmacy for clonazepam 0.125 mg ODT tablets (#90, 1 tab PO TID, 11 refills) b. Fax a prescription to the pharmacy for morphine 15 mg tablets (#10, 1 tab PO q4 PRN pain, 0 refills) c. Write a prescription for clobazam 10 mg tablets (#30, 1 tab PO QD, 5 refills) d. Electronically submit a prescription to the pharmacy for diazepam rectal gel (#1 box, insert 7.5 mg rectally PRN seizure, 1 refill)

DISPENSERS A pharmacist must dispense prescription to patient or member of the patient’s household A pharmacist may permit the delivery of a controlled substance by an authorized delivery person or by mail if records retained for 2 years of: Name, address, and date of birth or age of recipient Pharmacy must send record of prescription fill to Texas Prescription Monitoring Program (PMP) by electronic transfer no later than next business day Effective September 1, 2017 Previously submitted within 7 days of dispense Pharmacy must be registered with DEA using DEA Form 224 and renewed every 3 years utilizing DEA Form 224a

DISPENSERS Prescription label requirements Date of filling Pharmacy name and address Prescription number Name of patient Name of prescriber Directions for use Cautionary statements, if applicable Central fill pharmacy information, if applicable “CAUTION: Federal law prohibits the transfer of this drug to any person other than the patient for whom it was prescribed.”

VERBAL CHANGES FOR SCHEDULE II The following items cannot be modified: Name of the patient Name of the medication Name of the prescriber Date of the prescription Other items can be changed if the pharmacist: Contacts prescriber and obtains verbal authorization for change Documents date of prescriber consultation, change that was authorized, name or initials of individual granting authorization, initials of the pharmacist

NALOXONE STANDING ORDER Effective August 2016, Texas pharmacists can dispense and administer opioid antagonist (including necessary supplies) via physician-signed standing order if: Active license and in good standing with TSBP Completed 1-hour Texas accredited course provided by Accreditation Council for Pharmacy Education (ACPE) approved provider in coordination with Texas Pharmacy Association Standing order maintained at pharmacy

Jim’s father dropped off the below handsigned prescription to the TCH Outpatient Pharmacy. Will it be dispensed? a. Yes b. No Jim Smith 8 y/o 248 Magnolia Dr. Houston, TX 77701 04/02/18 Hydrocodoneacetaminophen 7.5 mg-325 mg/15 mL #60 (sixty) mL 5 mL PO q6 PRN severe pain 0 Dr. Zebra
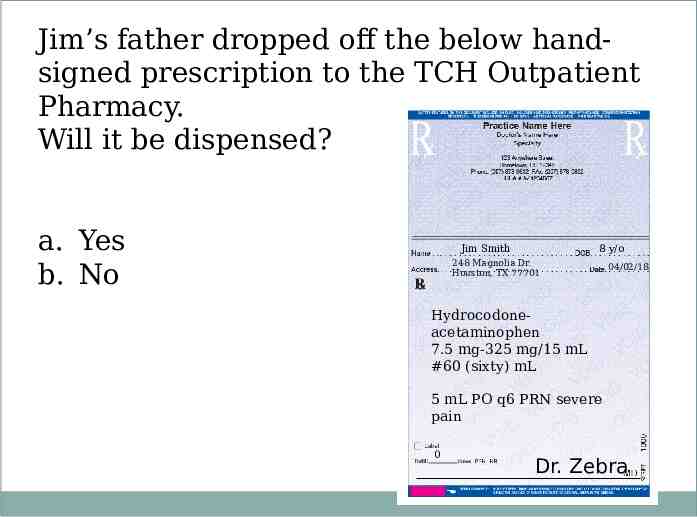
Jim’s father dropped off the below handsigned prescription to the TCH Outpatient Pharmacy. Will it be dispensed now? a. Yes b. No Jim Smith 248 Magnolia Dr. Houston, TX 77701 8 y/o 04/02/18 Hydrocodoneacetaminophen 7.5 mg-325 mg/15 mL #60 mL 5 mL PO q6 PRN severe pain Dr. Zebra 0

EMERGENCY PROCEDURES (CIII-V) Governor-declared state of disaster Notification received from executive director of Texas State Board of Pharmacy Pharmacist can dispense up to 30-day supply of prescription medications, other than schedule II, without prescriber authorization Refilling schedule III-V Pharmacist can exercise professional judgment in refilling without authorization of prescriber if: Failure may result in therapy interruption or patient suffering Unable to reach practitioner after reasonable effort Quantity does not exceed 72-hour supply Pharmacist informs patient that refill is provided without authorization Pharmacist informs prescriber at earliest reasonable time

EMERGENCY PROCEDURES (CII) Schedule II emergency Pharmacist can dispense oral or telephonically communicated prescription from practitioner Prescribing practitioner must provide written or electronic prescription to dispensing pharmacist within 7 days of authorization to be attached to oral emergency prescription Partial filling Permissible if pharmacist unable to supply full quantity of schedule II medication Pharmacist must make notation of quantity supplied Remainder must be filled within 72 hours of partial fill, otherwise forfeited

DIVERSION Any act or deviation that removes prescription medication from lawful purpose into illicit drug traffic Texas Health and Safety Code 481.1285: Diversion of Controlled Substance Knowingly converts to the person’s own use or benefit a controlled substance to which the person has access by virtue of the person’s profession or employment Knowingly diverts to the unlawful use or benefit of another person a controlled substance to which the person has access by virtue of the person’s profession or employment

What is a common risk point of controlled substance diversion in health systems? a. b. c. d. e. Procurement Preparation Prescribing Administration Waste

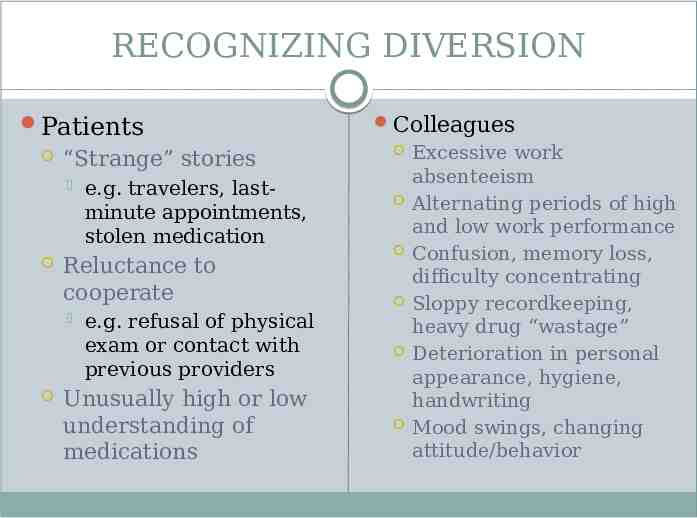
RECOGNIZING DIVERSION Patients “Strange” stories Reluctance to cooperate e.g. travelers, lastminute appointments, stolen medication e.g. refusal of physical exam or contact with previous providers Unusually high or low understanding of medications Colleagues Excessive work absenteeism Alternating periods of high and low work performance Confusion, memory loss, difficulty concentrating Sloppy recordkeeping, heavy drug “wastage” Deterioration in personal appearance, hygiene, handwriting Mood swings, changing attitude/behavior
PREVENTION METHODS Patients Obtain thorough history and complete physical examination Utilize patient medication agreements Protect prescription pads Collaborate with local pharmacies Maintain good medical practice standards Colleagues Demonstrate concern Immediately report theft/loss Support organization’s controlled substance diversion prevention program

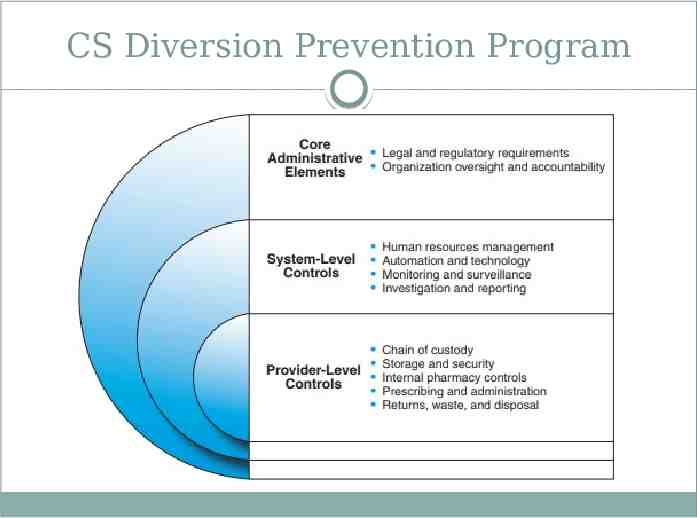
CS Diversion Prevention Program
PRESCRIPTION MONITORING PROGRAM State-administered data collection system used to gather prescription information Strongly endorsed by DEA as effective tool to detect pharmaceutical diversion and heighten awareness about prescription drug abuse http://www.pharmacy.texas.gov/PMP/

REFERENCES Brummond PW, Chen DF, Churchill WW, et al. ASHP Guidelines on Preventing Diversion of Controlled Substances. AM J Health Syst Pharm. 2017 Mar 1;74(5):325-348. Retrieved from http://www.ajhp.org/content/early/2016/12/22/ajhp160919. Centers for Disease Control and Prevention. Opioid Overdose. Retrieved from https://www.cdc.gov/drugoverdose/index.html. Drug Enforcement Administration. Drug Addiction in Health Care Professionals. Retrieved from https://www.deadiversion.usdoj.gov/pubs/brochures/drug hc.htm. Drug Enforcement Administration (2010). Pharmacist’s Manual. Retrieved from https://www.deadiversion.usdoj.gov/pubs/manuals/pharm2/index.html. Drug Enforcement Administration (2006). Practitioner’s Manual. Retrieved from https://www.deadiversion.usdoj.gov/pubs/manuals/pract/index.html. Drug Enforcement Administration (2016). Title 21 United States Code (USC) Controlled Substances Act. Retrieved from. https://www.deadiversion.usdoj.gov/21cfr/21usc/. Health and Safety Code, Title 16, Subtitle C, Chapter 481. Texas Controlled Substances Act. Retrieved from http:// www.statutes.legis.state.tx.us/Docs/HS/pdf/HS.481.pdf. Lipari RN, Williams M, Van Horn SL. Why do adults misuse prescription drugs? The CBHSQ Report. Rockville (MD): Substance Abuse and Mental Health Services Administration (US); 2013-. 2017 Jul 27. Retrieved from https://www.samhsa.gov/data/sites/default/files/report 3210/ShortReport-3210.html . Occupations Code, Title 3, Subtitle B, Chapter 157. Authority of Physician to Delegate Certain Medical Acts. Retrieved from http://www.statutes.legis.state.tx.us/Docs/OC/htm/OC.157.htm. Texas Pharmacy Association. Texas Pharmacist Naloxone Standing Order Application. Retrieved from http ://www.texaspharmacy.org/page/TXPHARMNALOX Texas State Board of Pharmacy . Texas Prescription Monitoring Program. Retrieved from http://www.pharmacy.texas.gov/PMP/. U.S. Department of Health and Human Services (2018, Feb 12). About the U.S. Opioid Epidemic. Retrieved from https://www.hhs.gov/opioids/about-the-epidemic/.







